Poster Session A
Rheumatoid arthritis (RA)
Session: (0380–0422) RA – Diagnosis, Manifestations, and Outcomes Poster I
0399: Differential Synovial Tissue Enrichment of Oxylipins and Their Mediators Across Rheumatoid Arthritis Trajectory
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- SP
Simone Perniola, MD, PhD
Division of Clinical Immunology – Fondazione Policlinico Universitario A. Gemelli IRCCS
Rome, ItalyDisclosure information not submitted.
Abstract Poster Presenter(s)
Simone Perniola1, Jessica Murillo-Saich2, Barbara Tolusso3, Clara Di Mario3, Marco Gessi4, Dario Bruno5, Luca Petricca6, Maria Rita Gigante6, Elisa Gremese5, Monica Guma7 and Stefano Alivernini8, 1Division of Clinical Immunology – Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, 2Department of Medicine, University of California San Diego, La Jolla, CA, 3Immunology Research Core Facility – Gemelli Science and Technology Park (GSTeP) - Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, 4Division of Pathology - Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, 5Division of Rheumatology, Institute of Rheumatology and Affine Sciences, School of Medicine, Catholic University of the Sacred Heart, Roma, Italy, 6Division of Rheumatology - Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, 7University of California San Diego, La Jolla, CA, 8Immunology Research Core Facility, Gemelli Science and Technology Park, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Division of Rheumatology - Fondazione Policlinico Universitario A. Gemelli IRCCS - Università Cattolica del Sacro Cuore, Rome, Italy
Background/Purpose: Synovial membrane represents the target tissue of Rheumatoid Arthritis (RA) inflammation. Our study aimed to perform a comprehensive quantification of oxylipins and their precursors in synovial tissue specimens of RA patients across disease trajectory.
Methods: 32 patients fulfilling the ACR/EULAR 2010 classification criteria underwent US-guided minimally invasive synovial tissue biopsy of the knee [n=16 patients with DAS28≥4.2 and n=16 patients in sustained (≥12 months) clinical (DAS28≤2.6) and ultrasound remission (Power Doppler negative)]. Oxylipins in synovial tissue were determined by liquid chromatography with tandem mass spectrometry (LC-MS-MS) and were classified into groups according to their precursor: arachidonic acid (AA), LA, ALA, DHA, EPA and DGLA. Moreover, each sample was processed for histology and the synovitis degree was assessed using H&E-based scoring by pathologist blinded on clinical characteristics. Statistical analysis was performed using MetaboAnalyst 5.0 and SPSS software v27.
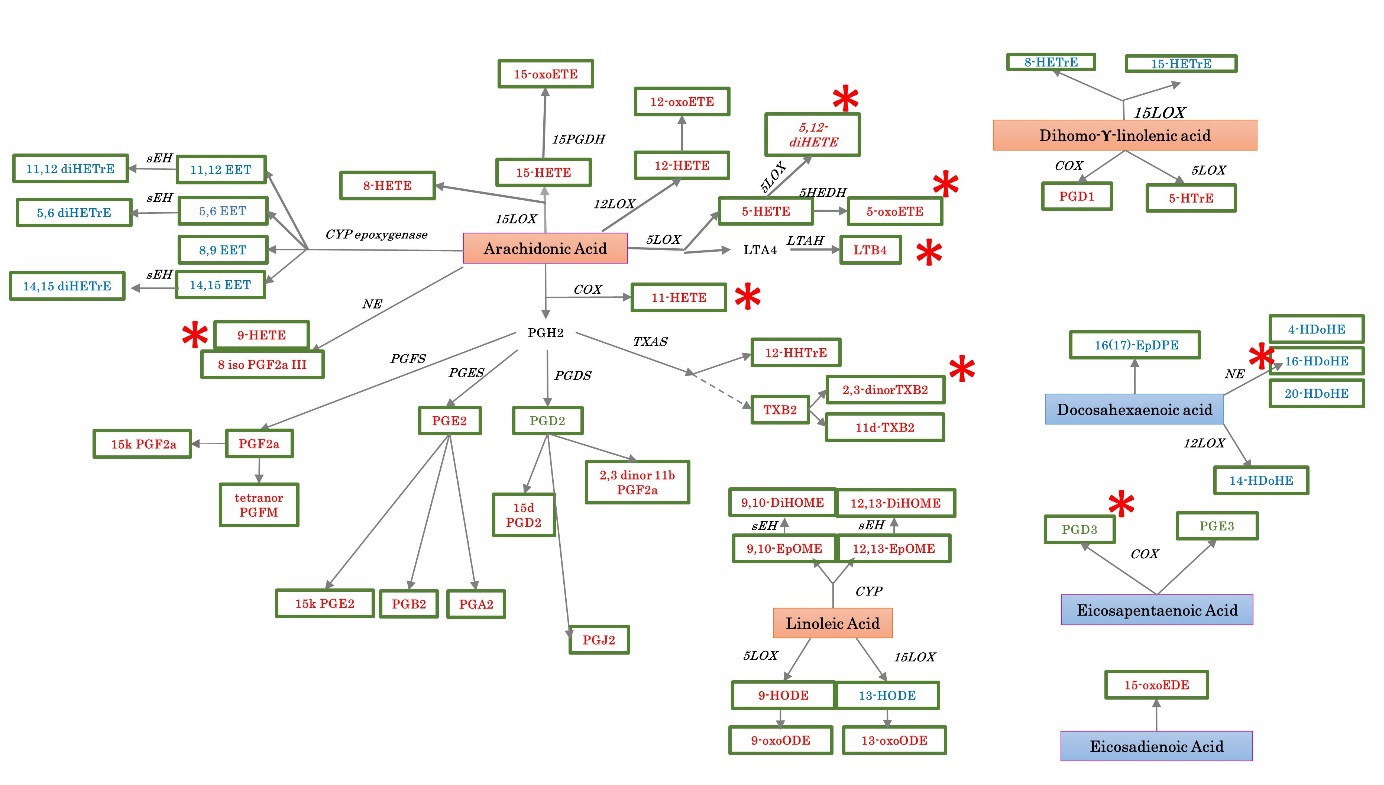
Results: Among the general panel, 84 different oxylipins were detected in at least one sample and 55 oxylipins remained after eliminating those with less than 50% across the samples (Figure 1). Stratifying RA patients based on the disease status, synovial tissue of active RA was significantly enriched of distinct specialized pro-resolving lipid mediators (SPMs) precursors such as free AA (p=0.0045), free adrenic acid (p=0.0080) and free docosahexaenoic acid (p=0.012) compared to synovial tissue of RA patients in sustained remission. Moreover, considering a threshold ≥4 of oxylipins expression, synovial tissue of active RA showed an 5LOX-derived oxylipins enrichment of 16.087 folds of 5,12-diHETE, 5.7419 folds of LTB4, TXA-derived 34.259 folds of 2,3 dinor TXB2, non-enzymatic -derived 6.192 folds of 16-HDoHE, 5.5097 folds of 9-HETE and, COX-derived oxylipins 4.8275 folds of 11-HETE compared to synovial tissue of RA patients in sustained clinical and ultrasound remission which was enriched 5.1589 folds of PGD3. Finally, the enrichment of pro-inflammatory SPMs was directly correlated with the histological synovitis degree (R2=0.473, p=0.006) particularly with the score of synovial hyperplasia (R2=0.472, p=0.006) and inflammatory infiltrate (R2=0.466, p=0.007).
Conclusion: Synovial tissue of RA patients shows a differential enrichment of oxylipins and their precursors which is contingent with the disease status directly mirroring the synovitis degree in terms of synovial hyperplasia and inflammatory infiltrate.
S. Perniola: None; J. Murillo-Saich: None; B. Tolusso: None; C. Di Mario: None; M. Gessi: None; D. Bruno: None; L. Petricca: None; M. Gigante: None; E. Gremese: None; M. Guma: None; S. Alivernini: None.
Background/Purpose: Synovial membrane represents the target tissue of Rheumatoid Arthritis (RA) inflammation. Our study aimed to perform a comprehensive quantification of oxylipins and their precursors in synovial tissue specimens of RA patients across disease trajectory.
Methods: 32 patients fulfilling the ACR/EULAR 2010 classification criteria underwent US-guided minimally invasive synovial tissue biopsy of the knee [n=16 patients with DAS28≥4.2 and n=16 patients in sustained (≥12 months) clinical (DAS28≤2.6) and ultrasound remission (Power Doppler negative)]. Oxylipins in synovial tissue were determined by liquid chromatography with tandem mass spectrometry (LC-MS-MS) and were classified into groups according to their precursor: arachidonic acid (AA), LA, ALA, DHA, EPA and DGLA. Moreover, each sample was processed for histology and the synovitis degree was assessed using H&E-based scoring by pathologist blinded on clinical characteristics. Statistical analysis was performed using MetaboAnalyst 5.0 and SPSS software v27.
Results: Among the general panel, 84 different oxylipins were detected in at least one sample and 55 oxylipins remained after eliminating those with less than 50% across the samples (Figure 1). Stratifying RA patients based on the disease status, synovial tissue of active RA was significantly enriched of distinct specialized pro-resolving lipid mediators (SPMs) precursors such as free AA (p=0.0045), free adrenic acid (p=0.0080) and free docosahexaenoic acid (p=0.012) compared to synovial tissue of RA patients in sustained remission. Moreover, considering a threshold ≥4 of oxylipins expression, synovial tissue of active RA showed an 5LOX-derived oxylipins enrichment of 16.087 folds of 5,12-diHETE, 5.7419 folds of LTB4, TXA-derived 34.259 folds of 2,3 dinor TXB2, non-enzymatic -derived 6.192 folds of 16-HDoHE, 5.5097 folds of 9-HETE and, COX-derived oxylipins 4.8275 folds of 11-HETE compared to synovial tissue of RA patients in sustained clinical and ultrasound remission which was enriched 5.1589 folds of PGD3. Finally, the enrichment of pro-inflammatory SPMs was directly correlated with the histological synovitis degree (R2=0.473, p=0.006) particularly with the score of synovial hyperplasia (R2=0.472, p=0.006) and inflammatory infiltrate (R2=0.466, p=0.007).
Conclusion: Synovial tissue of RA patients shows a differential enrichment of oxylipins and their precursors which is contingent with the disease status directly mirroring the synovitis degree in terms of synovial hyperplasia and inflammatory infiltrate.

Oxylipins observed in at least 50% of the samples by precursor and pathway are shown. Proinflammatory oxylipins are marked on red letters, while anti-inflammatory oxylipins are marked on blue letters. The n-3 polyunsaturated fatty acids (PUFAs) precursors are marked on red squares, while the n-6 PUFAs precursors are marked on blue squares. Those oxylipins with pro and anti-inflammatory reported roles are marked on green letters.
S. Perniola: None; J. Murillo-Saich: None; B. Tolusso: None; C. Di Mario: None; M. Gessi: None; D. Bruno: None; L. Petricca: None; M. Gigante: None; E. Gremese: None; M. Guma: None; S. Alivernini: None.



