Plenary Session
Epidemiology, health policy and outcomes
Session: Plenary II (1579–1584)
1582: Non-TNFi b/tsDMARDs vs. TNFi in Rheumatoid Arthritis-Interstitial Lung Disease: An Active-Comparator, New-User, Propensity Score Matched Study Using National Veterans Affairs Data
Monday, November 13, 2023
11:45 AM - 11:55 AM PT
Location: Exhibit Hall A-B
- BE
Bryant England, MD, PhD, RhMSUS
University of Nebraska Medical Center
Omaha, NE, United StatesDisclosure(s): Boehringer-Ingelheim: Consultant (Ongoing), Grant/Research Support (Ongoing)
Plenary Presenter(s)
Bryant England1, Joshua Baker2, Michael George2, Tate Johnson1, Yangyuna Yang1, Punyasha Roul1, Harlan Sayles1, Fang Yu1, Jorge Rojas Jr3, Brian Sauer4, Grant Cannon5, Jeffrey R Curtis6 and Ted R Mikuls7, 1University of Nebraska Medical Center, Omaha, NE, 2University of Pennsylvania, Philadelphia, PA, 3Puget Sound VA and University of Utah, Seattle, WA, 4Salt Lake City VA/University of Utah, Salt Lake City, UT, 5University of Utah and Salt Lake City VA, Salt Lake City, UT, 6Division of Clinical Immunology and Rheumatology, University of Alabama, Birmingham, AL, 7Division of Rheumatology and Immunology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: There is a paucity of data to guide biologic and JAKi DMARD selection in rheumatoid arthritis-interstitial lung disease (RA-ILD), with some reports of higher mortality with TNFi use in this population (Druce et al. RMD Open, 2017). We performed a real-world pharmacoepidemiologic study of non-TNFi/JAKi vs. TNFi in RA-ILD using the Target Trial Emulation framework (Hernan et al. JAMA, 2022).
Methods: We performed an active-comparator, new-user study of patients with RA-ILD initiating TNFi or non-TNFi biologic/JAKi in the Veterans Health Administration (VA) between 2006 and 2018. Receipt of ILD-focused therapies (e.g., mycophenolate, antifibrotics) was an exclusion. RA-ILD was identified using validated administrative algorithms requiring multiple RA and ILD diagnostic codes (PPV >70%). TNFi and non-TNFi/JAKi initiators were 1:1 propensity score (PS) caliper-matched using calendar time-specific PS models (2006-2010, 2011-2015, 2016-2018) that included demographics, healthcare utilization, comorbidities, and several RA- and ILD-related factors (including pre-treatment forced vital capacity [FVC]) obtained from EHR and administrative data (Figure 1). The primary outcome was a composite of time to respiratory-related hospitalization (by VA and Medicare data linkage) or death (by National Death Index linkage) using Cox regression models following an intention-to-treat analysis approach. Secondary outcomes were respiratory hospitalization and death (all-cause, respiratory). Outcomes were assessed over 3-year (primary) and 1-year (secondary) follow-up periods. Sensitivity analyses were performed among modified cohorts requiring: 1) prior biologic/JAKi treatment, 2) non-missing pre-treatment FVC values, or 3) additional ILD classification criteria.
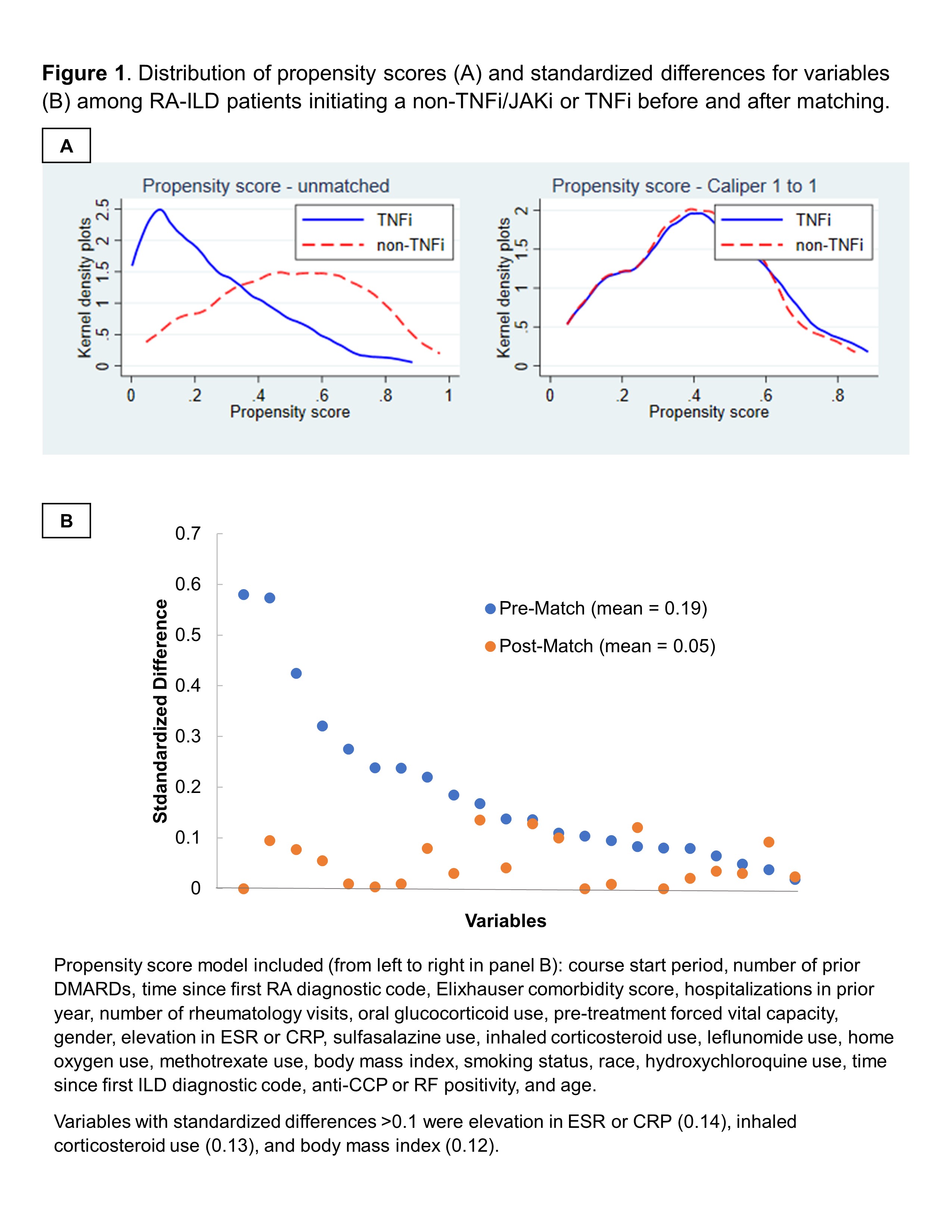
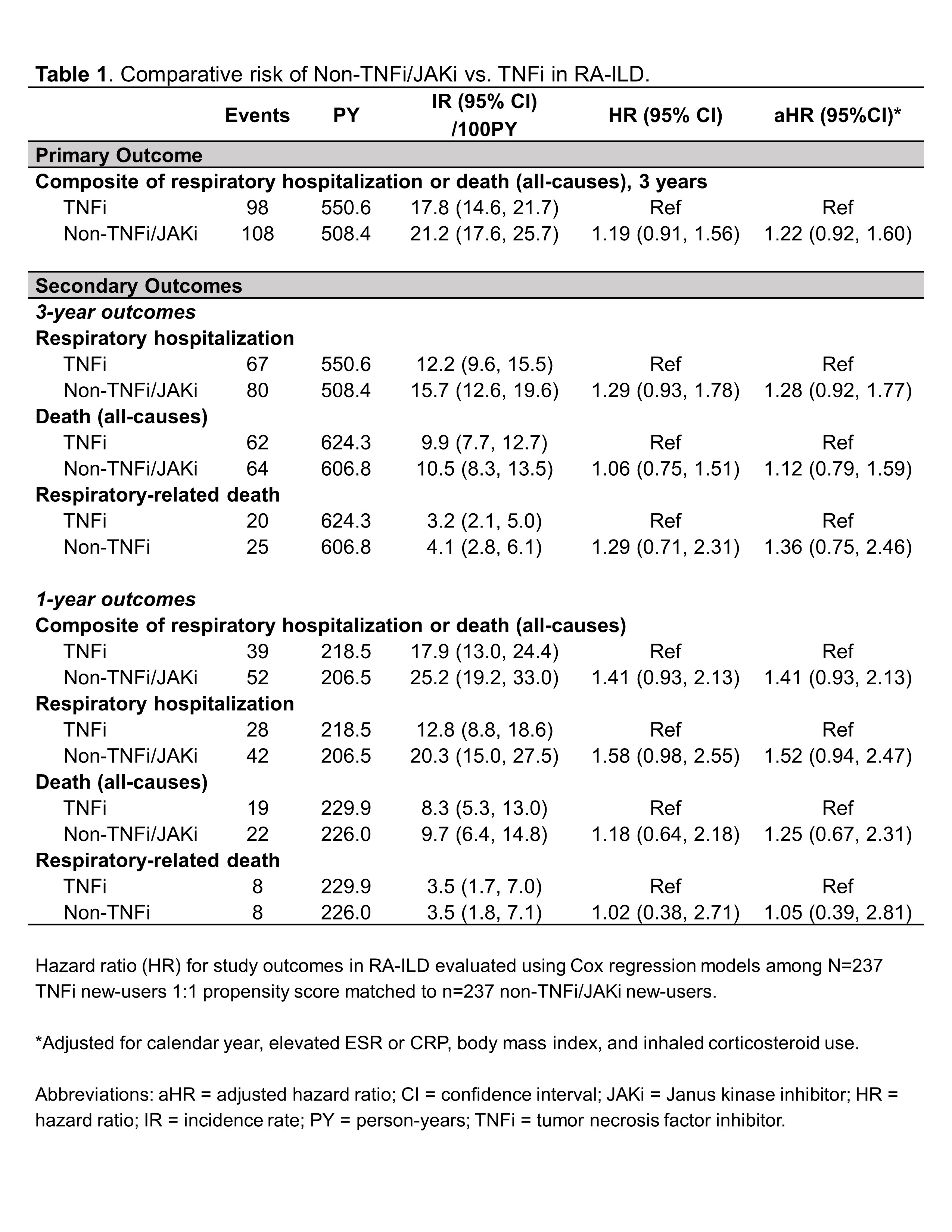
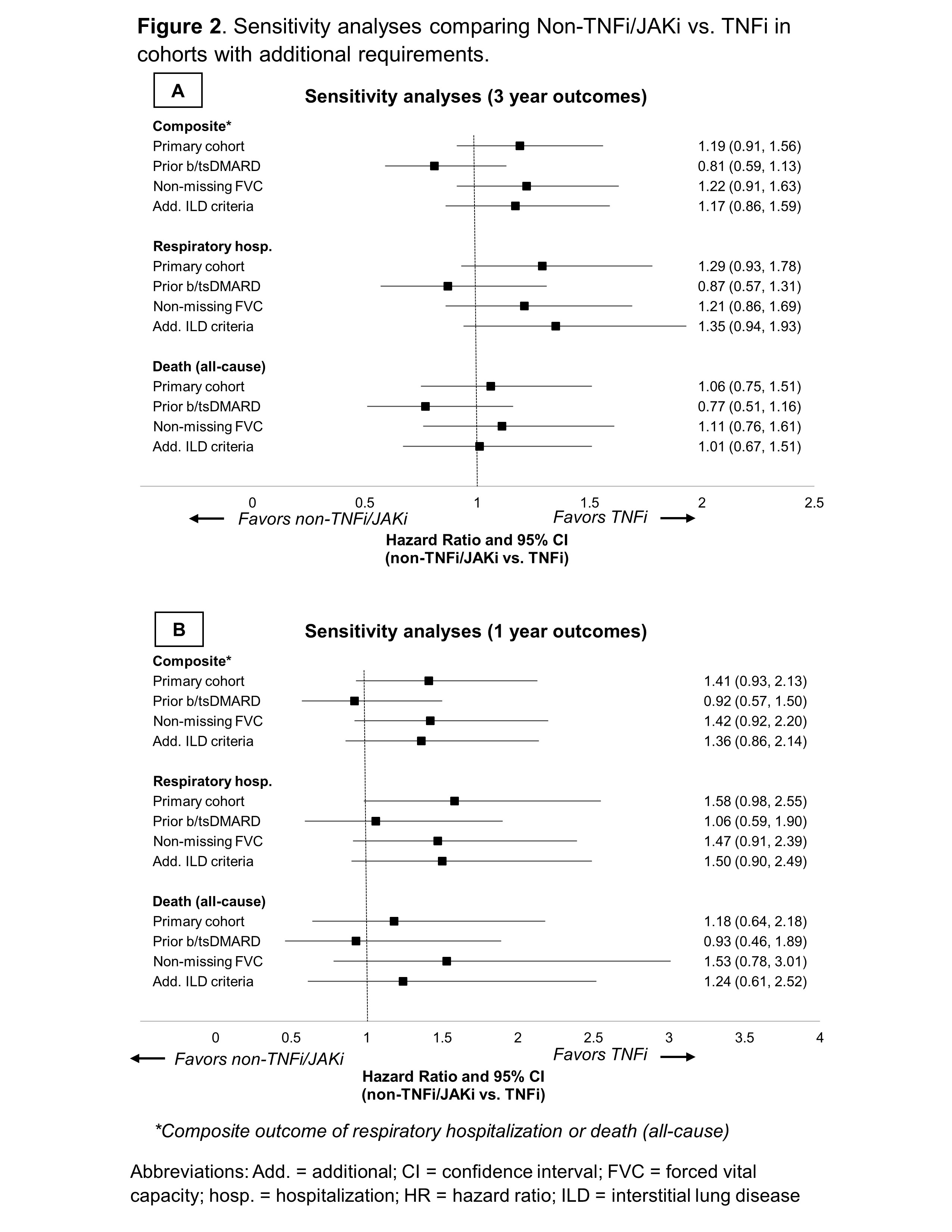
Results: Among 1,046 RA-ILD patients fulfilling eligibility criteria (n=704 TNFi), 237 TNFi initiators were matched to 237 non-TNFi/JAKi initiators in the primary analyses (mean age 68 years, 92% male). Mean standardized differences of variables in the PS model improved from 0.19 to 0.05 after matching, though few variables remained modestly imbalanced (Figure 1). Adalimumab (51%) and etanercept (37%) were the most frequent TNFi while rituximab (53%) and abatacept (28%) were the most frequent non-TNFi/JAKi. There was no significant difference in the primary outcome between non-TNFi/JAKi vs. TNFi (HR 1.19 [0.91, 1.56]; aHR 1.22 [0.92, 1.60]; Table 1). There were also no significant differences in respiratory hospitalization, all-cause mortality, or respiratory-related death (Table 1). In sensitivity analyses with modified cohort eligibility requirements, no significant differences in outcomes were observed between non-TNFi/JAKi and TNFi (Figure 2).
Conclusion: In this national VA, PS-matched study using the Target Trial Emulation framework, we did not observe significant differences in the risk of respiratory hospitalization or death between RA-ILD patients initiating non-TNFi/JAKi vs. TNFi. Our findings do not support systematic avoidance of TNFi in RA-ILD. Comparative data from clinical trials are needed to further guide these routine clinical decisions.
B. England: Boehringer-Ingelheim, 2, 5; J. Baker: CorEvitas, 2, Cumberland Pharma, 2, Horizon Pharmaceuticals, 5; M. George: AbbVie/Abbott, 2, GlaxoSmithKlein(GSK), 5, Janssen, 5; T. Johnson: None; Y. Yang: None; P. Roul: None; H. Sayles: None; F. Yu: None; J. Rojas Jr: None; B. Sauer: None; G. Cannon: None; J. Curtis: AbbVie, 2, 5, Amgen, 2, 5, BMS, 2, 5, Corrona, 2, 5, Crescendo, 2, 5, Genentech, 2, 5, Janssen, 2, 5, Pfizer, 2, 5, Roche, 2, 5, UCB Pharma, 2, 5; T. Mikuls: Elsevier, 9, Horizon Therapeutics, 2, 5, Pfizer, 2, Sanofi, 2, UCB Pharma, 2, Wolters Kluwer Health (UpToDate), 9.
Background/Purpose: There is a paucity of data to guide biologic and JAKi DMARD selection in rheumatoid arthritis-interstitial lung disease (RA-ILD), with some reports of higher mortality with TNFi use in this population (Druce et al. RMD Open, 2017). We performed a real-world pharmacoepidemiologic study of non-TNFi/JAKi vs. TNFi in RA-ILD using the Target Trial Emulation framework (Hernan et al. JAMA, 2022).
Methods: We performed an active-comparator, new-user study of patients with RA-ILD initiating TNFi or non-TNFi biologic/JAKi in the Veterans Health Administration (VA) between 2006 and 2018. Receipt of ILD-focused therapies (e.g., mycophenolate, antifibrotics) was an exclusion. RA-ILD was identified using validated administrative algorithms requiring multiple RA and ILD diagnostic codes (PPV >70%). TNFi and non-TNFi/JAKi initiators were 1:1 propensity score (PS) caliper-matched using calendar time-specific PS models (2006-2010, 2011-2015, 2016-2018) that included demographics, healthcare utilization, comorbidities, and several RA- and ILD-related factors (including pre-treatment forced vital capacity [FVC]) obtained from EHR and administrative data (Figure 1). The primary outcome was a composite of time to respiratory-related hospitalization (by VA and Medicare data linkage) or death (by National Death Index linkage) using Cox regression models following an intention-to-treat analysis approach. Secondary outcomes were respiratory hospitalization and death (all-cause, respiratory). Outcomes were assessed over 3-year (primary) and 1-year (secondary) follow-up periods. Sensitivity analyses were performed among modified cohorts requiring: 1) prior biologic/JAKi treatment, 2) non-missing pre-treatment FVC values, or 3) additional ILD classification criteria.
Results: Among 1,046 RA-ILD patients fulfilling eligibility criteria (n=704 TNFi), 237 TNFi initiators were matched to 237 non-TNFi/JAKi initiators in the primary analyses (mean age 68 years, 92% male). Mean standardized differences of variables in the PS model improved from 0.19 to 0.05 after matching, though few variables remained modestly imbalanced (Figure 1). Adalimumab (51%) and etanercept (37%) were the most frequent TNFi while rituximab (53%) and abatacept (28%) were the most frequent non-TNFi/JAKi. There was no significant difference in the primary outcome between non-TNFi/JAKi vs. TNFi (HR 1.19 [0.91, 1.56]; aHR 1.22 [0.92, 1.60]; Table 1). There were also no significant differences in respiratory hospitalization, all-cause mortality, or respiratory-related death (Table 1). In sensitivity analyses with modified cohort eligibility requirements, no significant differences in outcomes were observed between non-TNFi/JAKi and TNFi (Figure 2).
Conclusion: In this national VA, PS-matched study using the Target Trial Emulation framework, we did not observe significant differences in the risk of respiratory hospitalization or death between RA-ILD patients initiating non-TNFi/JAKi vs. TNFi. Our findings do not support systematic avoidance of TNFi in RA-ILD. Comparative data from clinical trials are needed to further guide these routine clinical decisions.

Figure 1. Distribution of propensity scores (A) and standardized differences for variables (B) among RA-ILD patients initiating a non-TNFi/JAKi or TNFi before and after matching.

Table 1. Comparative risk of Non-TNFi/JAKi vs .TNFi in RA-ILD.

Figure 2. Sensitivity analyses comparing Non-TNFi/JAKi vs. TNFi in cohorts with additional requirements.
B. England: Boehringer-Ingelheim, 2, 5; J. Baker: CorEvitas, 2, Cumberland Pharma, 2, Horizon Pharmaceuticals, 5; M. George: AbbVie/Abbott, 2, GlaxoSmithKlein(GSK), 5, Janssen, 5; T. Johnson: None; Y. Yang: None; P. Roul: None; H. Sayles: None; F. Yu: None; J. Rojas Jr: None; B. Sauer: None; G. Cannon: None; J. Curtis: AbbVie, 2, 5, Amgen, 2, 5, BMS, 2, 5, Corrona, 2, 5, Crescendo, 2, 5, Genentech, 2, 5, Janssen, 2, 5, Pfizer, 2, 5, Roche, 2, 5, UCB Pharma, 2, 5; T. Mikuls: Elsevier, 9, Horizon Therapeutics, 2, 5, Pfizer, 2, Sanofi, 2, UCB Pharma, 2, Wolters Kluwer Health (UpToDate), 9.



