Plenary Session
Vasculitis
Session: Plenary I (0722–0726)
0725: Real-life Use of the PEXIVAS Reduced-dose Glucocorticoid Regimen in Granulomatosis with Polyangiitis and Microscopic Polyangiitis
Sunday, November 12, 2023
12:00 PM - 12:10 PM PT
Location: Exhibit Hall A-B
- SN
Sophie Nagle (she/her/hers)
Université Paris Saclay
Montreuil (93100), FranceDisclosure(s): No financial relationships with ineligible companies to disclose
Plenary Presenter(s)
Sophie Nagle1, Yann Nguyen2, Xavier Puéchal3, Dimitri Titeca-Beauport4, Thomas Crépin5, Rafik Mesbah6, Idris Boudhabhay7, Gregory Pugnet8, Juliette Woessner9, Roderau Outh10, Céline Lebas11, Antoine Néel12, alexandre Karras13, Eric Hachulla11, Benjamin Subran14, Philippe Kerschen15, Mathieu Gerfaud-Valentin16, Stéphane Vinzio17, Tiphaine Goulenok18, Raphael Borie19, Sébastien Humbert20, Yurdagul Uzunhan21, Sarah Melboucy-Belkhir22, Jean-Baptiste Gouin23, Mary-Jane Guerry24 and Benjamin Terrier25, 1AP-HP Cochin Hospital, Paris, France, 2Department of Internal Medicine, Hôpital Beaujon, AP-HP, Clichy, France., Montmorency, France, 3National Referral Center for Rare Systemic Autoimmune Diseases, Paris, France, 4CHU Amiens, Amiens, France, 5Amiens University Hospital, Amiens, France, 6Boulogne Hospital (CH), Boulogne, France, 7Necker University Hospital, Paris, France, 8CHU Toulouse Rangueil Service de Medecine Interne et Immunologie Clinique, Toulouse, France, 9Avignon Hospital (CH), Avignon, France, 10Perpignan Hospital (CH), Perpignan, France, 11Lille University Hospital, Lille, France, 12CHU Nantes, Nantes, France, 13HEGP - APHP, Paris, France, 14Croix Saint Simon Hospital, Paris, France, 15Department of Neurology, Luxembourg Hospital Center, Luxembourg City, Luxembourg, 16Lyon University Hospital, Lyon, France, 17Grenoble Hospital (CH), Grenoble, France, 18Assistance Publique Hopitaux de Paris, Paris, France, 19Bichat-Claude Bernard, Universite de Paris, Paris, France, 20Besançon University Hospital, Besançon, France, 21AP-HP, Bobigny, France, 22Saint Quentin Hospital, Saint-Quentin, France, 23Vannes Hospital (CH Bretagne Atlantique), Vannes, France, 24Valenciennes Hospital (CH), Valenciennes, France, 25Department of Internal Medicine, Hôpital Cochin, AP-HP, Paris, France
Background/Purpose: Glucocorticoids (GCs) in combination with rituximab (RTX) or cyclophosphamide are the cornerstone of treatment for patients with severe granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). GCs are associated with adverse effects, including serious infections. The PEXIVAS trial demonstrated non-inferiority of reduced-dose GC regimen compared to standard dose for the incidence of death or end-stage kidney disease (ESKD), with a significant reduction in serious infections at one year. However, the primary endpoint did not include disease progression or relapse, the majority of patients received cyclophosphamide as induction therapy, and subgroup analysis showed a trend towards an increased risk of death or ESKD in RTX-treated patients. We aimed to evaluate the efficacy and safety of the reduced-dose GC regimen in a real-world setting.
Methods: We conducted a retrospective, multicentre study comparing the PEXIVAS reduced-dose GC regimen with a standard regimen in patients with severe GPA or PAM flare between January 2018 and April 2022. The primary composite endpoint included the occurrence of death, ESKD, progression before remission requiring treatment modification or relapse, whichever occurred first. Factors associated with the occurrence of the primary endpoint and of death or ESKD (i.e. the PEXIVAS endpoint) were estimated using univariate and multivariate Cox models. In a sensitivity analysis, patients treated with reduced-dose and standard-dose GCs were compared after matching on a propensity score.
Results: Of the 234 patients enrolled (93 MPA and 148 GPA), 126 (53.8%) received a reduced GC regimen and 108 (46.2%) received a standard regimen. The primary endpoint occurred in 62/234 (26.5%) of patients during the first year of follow-up: 33.3% of patients on the reduced dose versus 18.5% on the standard dose (p=0.016).
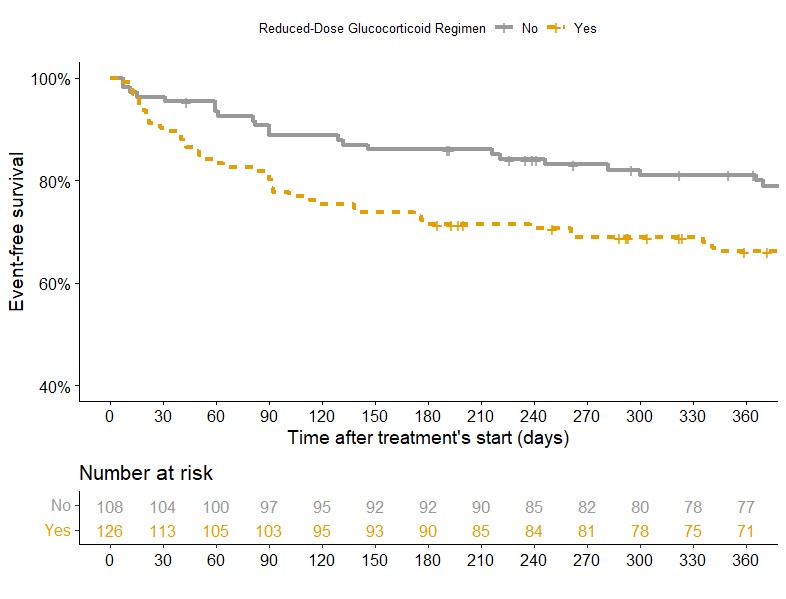
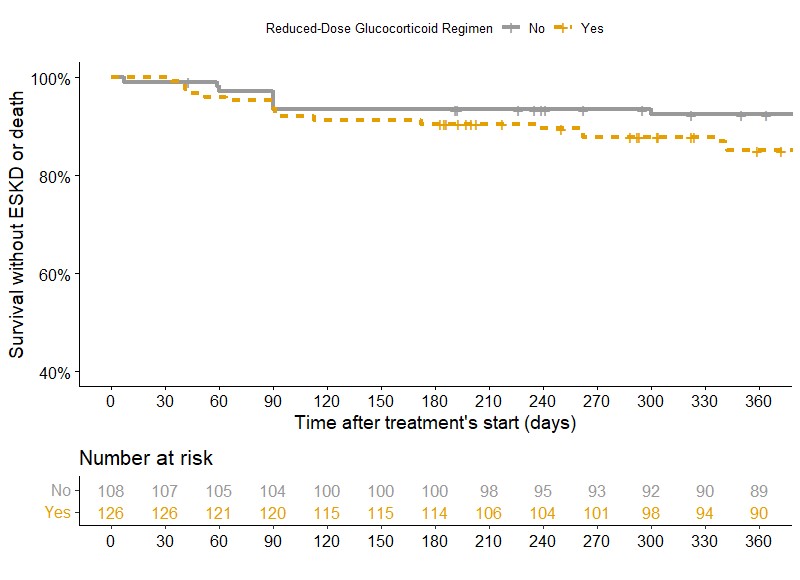
In multivariate analysis, a reduced GC regimen was significantly associated with the occurrence of the endpoint compared to a standard regimen (HR 1.72; 95%CI 1.08-2.74) (Figure 1), but was not associated with an increased risk of death or ESKD (HR 1.62; 95%CI 0.82-3.19) (Figure 2). There was no significant difference in serious infections at 1 year (20.6% vs 15.7%, p=0.427).
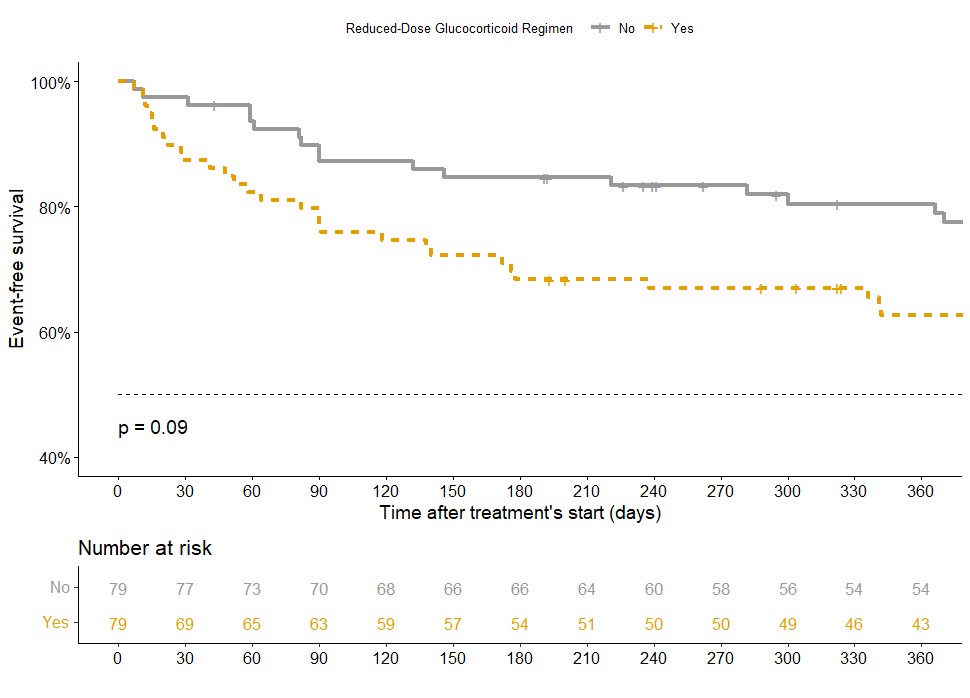
After propensity score matching, the reduced-dose GC regimen tended to be more likely to meet the primary endpoint than the standard regimen (HR 1.57; 95%CI 0.93-2.64) (Figure 3). In the subgroup of patients treated with the reduced-dose GC regimen, patients with creatinine levels above 300 μmol/L were more likely to meet the primary endpoint (RR 2.14; 95% CI 1.14-4.03). Similarly, in the subgroup of patients treated with RTX, the reduced-dose GC regimen tended to be more likely to achieve the primary endpoint (HR 1.61; 95% CI 0.94-2.77) and was more likely to meet death or ESKD (HR 2.42; 95%CI 1.04-5.66).
Conclusion: In patients with severe GPA or MPA, the reduced-dose GC regimen was associated with an increased risk of death, ESKD, progression before remission requiring treatment modification or relapse. This risk was even greater in patients with creatinine levels above 300 μmol/L and in those treated with RTX as induction therapy.
S. Nagle: None; Y. Nguyen: None; X. Puéchal: None; D. Titeca-Beauport: None; T. Crépin: None; R. Mesbah: None; I. Boudhabhay: None; G. Pugnet: None; J. Woessner: None; R. Outh: None; C. Lebas: None; A. Néel: None; a. Karras: AstraZeneca, 6, GlaxoSmithKlein(GSK), 4, Novartis, 2, Pfizer, 6; E. Hachulla: None; B. Subran: None; P. Kerschen: None; M. Gerfaud-Valentin: None; S. Vinzio: None; T. Goulenok: None; R. Borie: None; S. Humbert: None; Y. Uzunhan: None; S. Melboucy-Belkhir: None; J. Gouin: None; M. Guerry: None; B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2.
Background/Purpose: Glucocorticoids (GCs) in combination with rituximab (RTX) or cyclophosphamide are the cornerstone of treatment for patients with severe granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). GCs are associated with adverse effects, including serious infections. The PEXIVAS trial demonstrated non-inferiority of reduced-dose GC regimen compared to standard dose for the incidence of death or end-stage kidney disease (ESKD), with a significant reduction in serious infections at one year. However, the primary endpoint did not include disease progression or relapse, the majority of patients received cyclophosphamide as induction therapy, and subgroup analysis showed a trend towards an increased risk of death or ESKD in RTX-treated patients. We aimed to evaluate the efficacy and safety of the reduced-dose GC regimen in a real-world setting.
Methods: We conducted a retrospective, multicentre study comparing the PEXIVAS reduced-dose GC regimen with a standard regimen in patients with severe GPA or PAM flare between January 2018 and April 2022. The primary composite endpoint included the occurrence of death, ESKD, progression before remission requiring treatment modification or relapse, whichever occurred first. Factors associated with the occurrence of the primary endpoint and of death or ESKD (i.e. the PEXIVAS endpoint) were estimated using univariate and multivariate Cox models. In a sensitivity analysis, patients treated with reduced-dose and standard-dose GCs were compared after matching on a propensity score.
Results: Of the 234 patients enrolled (93 MPA and 148 GPA), 126 (53.8%) received a reduced GC regimen and 108 (46.2%) received a standard regimen. The primary endpoint occurred in 62/234 (26.5%) of patients during the first year of follow-up: 33.3% of patients on the reduced dose versus 18.5% on the standard dose (p=0.016).
In multivariate analysis, a reduced GC regimen was significantly associated with the occurrence of the endpoint compared to a standard regimen (HR 1.72; 95%CI 1.08-2.74) (Figure 1), but was not associated with an increased risk of death or ESKD (HR 1.62; 95%CI 0.82-3.19) (Figure 2). There was no significant difference in serious infections at 1 year (20.6% vs 15.7%, p=0.427).
After propensity score matching, the reduced-dose GC regimen tended to be more likely to meet the primary endpoint than the standard regimen (HR 1.57; 95%CI 0.93-2.64) (Figure 3). In the subgroup of patients treated with the reduced-dose GC regimen, patients with creatinine levels above 300 μmol/L were more likely to meet the primary endpoint (RR 2.14; 95% CI 1.14-4.03). Similarly, in the subgroup of patients treated with RTX, the reduced-dose GC regimen tended to be more likely to achieve the primary endpoint (HR 1.61; 95% CI 0.94-2.77) and was more likely to meet death or ESKD (HR 2.42; 95%CI 1.04-5.66).
Conclusion: In patients with severe GPA or MPA, the reduced-dose GC regimen was associated with an increased risk of death, ESKD, progression before remission requiring treatment modification or relapse. This risk was even greater in patients with creatinine levels above 300 μmol/L and in those treated with RTX as induction therapy.

Kaplan-Meier survival curve comparing occurence of primary endpoint at 12 months between patients treated with reduced-dose GC regimen (Yes: orange) and patients treated with standard-dose regimen (No: grey) during the first year of follow-up

Kaplan-Meier survival curve comparing occurence of death or ESKD at 12 months between patients treated with reduced-dose GC regimen (Yes: orange) and patients treated with standard-dose regimen (No: grey) during the first year of follow-up

Kaplan-Meier survival curve comparing occurence of primary endpoint at 12 months between patients treated with reduced-dose GC regimen (Yes: orange) and patients treated with standard-dose regimen (No: grey) during the first year of follow-up, after matching with a propensity score
S. Nagle: None; Y. Nguyen: None; X. Puéchal: None; D. Titeca-Beauport: None; T. Crépin: None; R. Mesbah: None; I. Boudhabhay: None; G. Pugnet: None; J. Woessner: None; R. Outh: None; C. Lebas: None; A. Néel: None; a. Karras: AstraZeneca, 6, GlaxoSmithKlein(GSK), 4, Novartis, 2, Pfizer, 6; E. Hachulla: None; B. Subran: None; P. Kerschen: None; M. Gerfaud-Valentin: None; S. Vinzio: None; T. Goulenok: None; R. Borie: None; S. Humbert: None; Y. Uzunhan: None; S. Melboucy-Belkhir: None; J. Gouin: None; M. Guerry: None; B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2.



