Poster Session A
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: (0345–0379) Pediatric Rheumatology – Clinical Poster I: JIA
0362: Adverse Childhood Experiences Are Associated with Disease Outcomes in Youth with Juvenile Idiopathic Arthritis
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- DB
Danielle Bullock, MD, MPH
University of Minnesota
Minneapolis, MN, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Danielle Bullock1, Ilir Agalliu2, Evin Rothschild2, Kaveh Ardalan3, Erica Lawson4, Natoshia Cunningham5, William Soulsby4, Nicole Reitz6, Laura Bouslaugh7, Anna Diggs8, Elizabeth Kessler9, Sara Kramer1, Bhupinder Nahal4, Cassandra Davis10, Ashley Kemp1, Riley O'Connor1, Tiffany Chinn4, Kathy Nguyen4, Paige Hill1 and Tamar Rubinstein11, 1University of Minnesota, Minneapolis, MN, 2Albert Einstein College of Medicine, Bronx, NY, 3Duke University School of Medicine, Durham, NC, 4University of California San Francisco, San Francisco, CA, 5Michigan State University, Grand Rapids, MI, 6CARRA, Allendale, MI, 7CARRA, Washington, DC, 8CARRA, Tipton, MO, 9Spectrum Health, Grand Rapids, MI, 10University of Utah, Salt Lake City, UT, 11Albert Einstein College of Medicine, Children's Hospital at Montefiore, White Plains, NY
Background/Purpose: Adverse childhood events (ACEs) are common in children. ACEs are various stressors, including, but not limited to parental incarceration or food and housing insecurity. Our previous work showed that children with arthritis were more likely to be affected by ACEs than healthy children and children with other chronic diseases. To gain a better understanding of the relationship between ACEs and disease outcomes in Juvenile Idiopathic Arthritis (JIA), we screened JIA enrollees from the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry for ACEs.
Methods: Patients and their families who met inclusion criteria and consented to the study received an ACE screen at CARRA study visits. For participants < 18 years, parents completed the screen; patients ≥18 years completed ACE screen. Demographic and JIA disease data collected in the CARRA Registry were linked to ACE screen data. ACE scores were categorized as 0, 1, 2-3, and ≥4. JIA outcomes examined included disease activity (JADAS), physical impairment (CHAQ and PROMIS Mobility), physician global assessment, patient/parent global assessment, pain intensity scale, PROMIS Pain Interference, and PROMIS Pediatric Global Health Index. Bivariate analyses were conducted to assess for associations between categorical ACE scores, demographics, clinical characteristics and outcomes. Chi-square and Fisher's exact tests were used for categorical variables and Kruskall-Wallis tests were used for continuous variables.
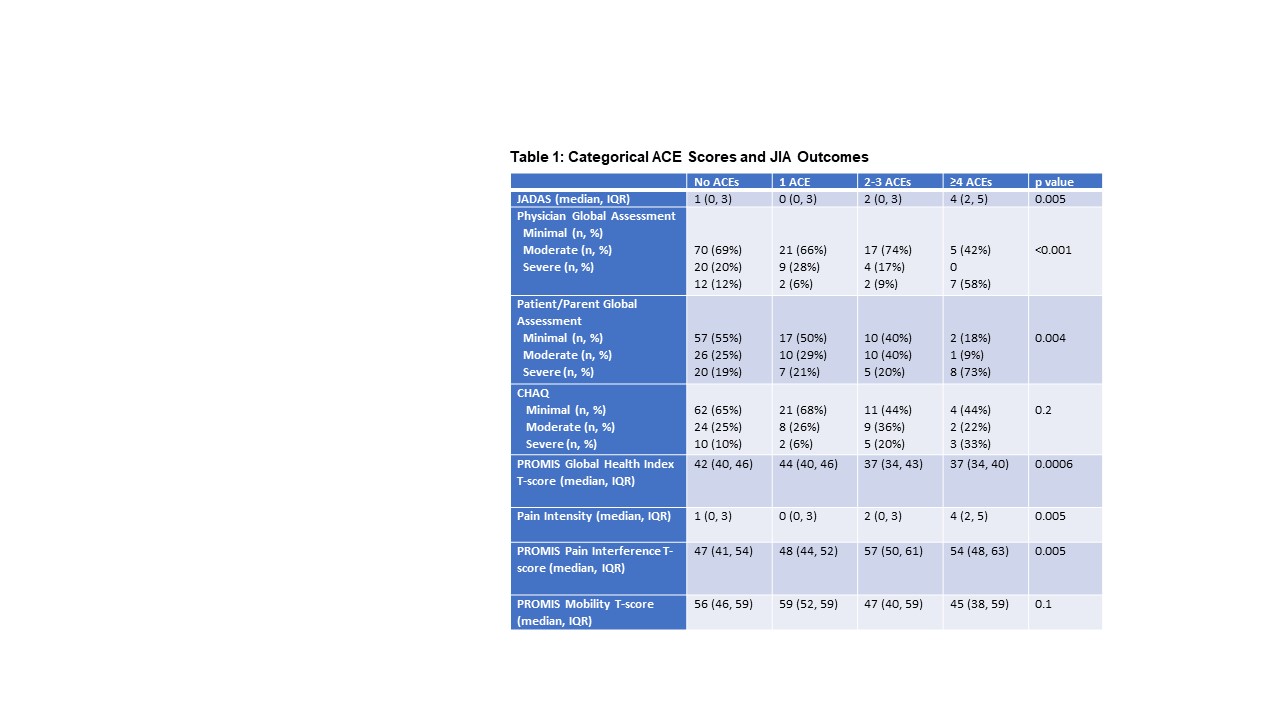
Results: Among 209 participants screened for ACEs, 130 (62%) had none, 38 (18%) had 1, 28 (13%) had 2-3, and 13 (6%) had ≥4. Categorical ACE scores differed by age (p< 0.001), race/ethnicity (p=0.002), household income (p< 0.001), parental education level (p=0.01), health insurance status (p< 0.001), Area of Deprivation Index national ranking (p=0.01), and estimated poverty rate by zipcode (p=0.002). Associations between categorical ACE scores and JIA outcomes are described in Table 1. Higher ACE scores were associated with poorer measures of disease activity (JADAS, physician and patient/parent global assessments), higher pain intensity and pain interference, and a poorer PROMIS Global Health Index. While associations with CHAQ and PROMIS Mobility scores did not meet significance, higher proportions of impaired youth were observed among youth with ACE scores of 2-3 and ≥4.
Conclusion: Higher ACE scores were associated with worse JIA outcome measures including disease activity, pain, global health, and patient/parent and physician global assessments. Further work needs to be done to see whether addressing stress around ACEs in youth with JIA can improved disease outcomes.
D. Bullock: None; I. Agalliu: None; E. Rothschild: None; K. Ardalan: Cure JM Foundation (grant funding to support clinical trial design with ReveraGen BioPharma, but no direct funding from ReveraGen), 12, Cure JM Foundation grant funding to support clinical trial design with ReveraGen BioPharma, but no direct funding from ReveraGen; E. Lawson: Pfizer, 5; N. Cunningham: DoD, 12, DoD Transformative Vision Award (starting Oct 2023) and CARRA Transdisciplinary Research Grant (NCE). I also have other current funding support.; W. Soulsby: None; N. Reitz: None; L. Bouslaugh: None; A. Diggs: None; E. Kessler: None; S. Kramer: None; B. Nahal: None; C. Davis: None; A. Kemp: None; R. O'Connor: None; T. Chinn: None; K. Nguyen: None; P. Hill: None; T. Rubinstein: None.
Background/Purpose: Adverse childhood events (ACEs) are common in children. ACEs are various stressors, including, but not limited to parental incarceration or food and housing insecurity. Our previous work showed that children with arthritis were more likely to be affected by ACEs than healthy children and children with other chronic diseases. To gain a better understanding of the relationship between ACEs and disease outcomes in Juvenile Idiopathic Arthritis (JIA), we screened JIA enrollees from the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry for ACEs.
Methods: Patients and their families who met inclusion criteria and consented to the study received an ACE screen at CARRA study visits. For participants < 18 years, parents completed the screen; patients ≥18 years completed ACE screen. Demographic and JIA disease data collected in the CARRA Registry were linked to ACE screen data. ACE scores were categorized as 0, 1, 2-3, and ≥4. JIA outcomes examined included disease activity (JADAS), physical impairment (CHAQ and PROMIS Mobility), physician global assessment, patient/parent global assessment, pain intensity scale, PROMIS Pain Interference, and PROMIS Pediatric Global Health Index. Bivariate analyses were conducted to assess for associations between categorical ACE scores, demographics, clinical characteristics and outcomes. Chi-square and Fisher's exact tests were used for categorical variables and Kruskall-Wallis tests were used for continuous variables.
Results: Among 209 participants screened for ACEs, 130 (62%) had none, 38 (18%) had 1, 28 (13%) had 2-3, and 13 (6%) had ≥4. Categorical ACE scores differed by age (p< 0.001), race/ethnicity (p=0.002), household income (p< 0.001), parental education level (p=0.01), health insurance status (p< 0.001), Area of Deprivation Index national ranking (p=0.01), and estimated poverty rate by zipcode (p=0.002). Associations between categorical ACE scores and JIA outcomes are described in Table 1. Higher ACE scores were associated with poorer measures of disease activity (JADAS, physician and patient/parent global assessments), higher pain intensity and pain interference, and a poorer PROMIS Global Health Index. While associations with CHAQ and PROMIS Mobility scores did not meet significance, higher proportions of impaired youth were observed among youth with ACE scores of 2-3 and ≥4.
Conclusion: Higher ACE scores were associated with worse JIA outcome measures including disease activity, pain, global health, and patient/parent and physician global assessments. Further work needs to be done to see whether addressing stress around ACEs in youth with JIA can improved disease outcomes.

Bivariate relationships between catgerical ACE scores and JIA outcome variables. JADAS, clinical Juvenile Arthritis Disease Activity Score; CHAQ, Childhood Health Assessment Questionnaire; PROMIS Patient-Reported Outcomes Measurement Information System. Physician and Parent Global Assessments were measured by 10-cm visual analog scales with mild, moderate, and severe defined by 25%-ile, 50%-ile, and 75%ile.
D. Bullock: None; I. Agalliu: None; E. Rothschild: None; K. Ardalan: Cure JM Foundation (grant funding to support clinical trial design with ReveraGen BioPharma, but no direct funding from ReveraGen), 12, Cure JM Foundation grant funding to support clinical trial design with ReveraGen BioPharma, but no direct funding from ReveraGen; E. Lawson: Pfizer, 5; N. Cunningham: DoD, 12, DoD Transformative Vision Award (starting Oct 2023) and CARRA Transdisciplinary Research Grant (NCE). I also have other current funding support.; W. Soulsby: None; N. Reitz: None; L. Bouslaugh: None; A. Diggs: None; E. Kessler: None; S. Kramer: None; B. Nahal: None; C. Davis: None; A. Kemp: None; R. O'Connor: None; T. Chinn: None; K. Nguyen: None; P. Hill: None; T. Rubinstein: None.



