Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0582–0608) SLE – Treatment Poster I
0598: Litifilimab Modulates Type I IFN Biomarkers in Patients with SLE or CLE in the Phase 2 LILAC Study
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Richard Furie, MD
Chief of the Division of Rheumatology
Northwell Health
Manhasset, NY, NY, United StatesDisclosure(s): Biogen: Consultant (Ongoing), Grant/Research Support (Ongoing)
Abstract Poster Presenter(s)
Richard Furie1, Victoria Werth2, Eric Milliman3, Kyle Ferber3, Fergal Casey3, Roland Brown3, Denitza Raitcheva3, Jad Zoghbi3, Danielle Graham3, George Kong3, Youmna Lahoud3, Nathalie Franchimont4 and Catherine Barbey5, 1Northwell Health, Manhasset, NY, 2University of Pennsylvania and Corporal Michael J. Crescenz VAMC, Philadelphia, PA, 3Biogen, Cambridge, MA, 4Former employee of Biogen, Cambridge, MA, 5Biogen, Baar, Switzerland
Background/Purpose: Litifilimab is a humanized IgG1 mAb targeting BDCA2, a receptor expressed on plasmacytoid dendritic cells (pDCs), that negatively regulates the production of Type I IFN and proinflammatory chemokines and cytokines.1,2 In the Phase 2 LILAC study of litifilimab (NCT02847598), Part A (participants with SLE, active arthritis and rash) and Part B (participants with active CLE, with or without SLE) met their primary endpoints at Weeks (W) 24 and 16, respectively.1,2 We evaluated the effects of litifilimab on IFN gene signature (IFNGS) scores from whole blood samples, serum IFNα concentrations, and other serum cytokine concentrations in LILAC participants.3,4
Methods: Expression of 22-gene panel IFNGS scores and concentrations of IFNα and other cytokines were examined over time in the modified intent-to-treat population (Part A N=120, Part B N=132) with available data at baseline (BL) and ≥1 post-BL visit. Treatment effects were estimated using a mixed model repeated measures approach. In Part A, IFNα concentration changes over time were compared with W24 total active joint counts and CLASI-A scores (Spearman rank correlations) and SLE Responder Index (SRI-4) status. In Part B, Pearson correlations between percent changes in CLASI-A score and IFNGS score were estimated and the dose-response relationship was estimated using a previously described MCP-Mod method.2
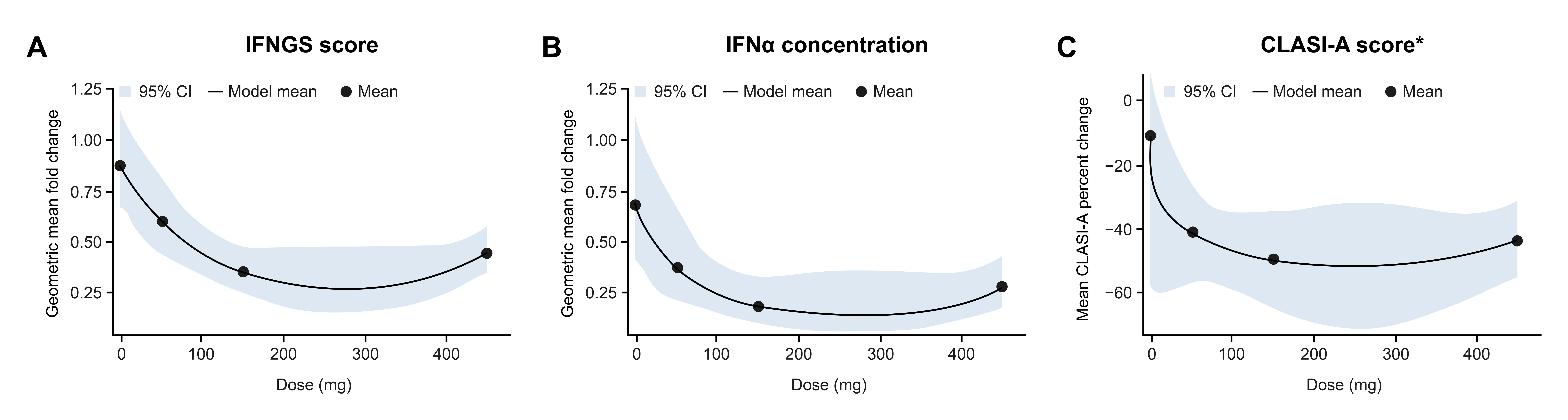
Results: As too few participants were IFNGS-low at BL, only data for the IFNGS-high subgroups are described. Litifilimab induced rapid, substantial, and sustained reductions in 22-gene IFNGS scores and IFNα concentrations that were greater (nominal P< 0.05) vs placebo (PBO) with litifilimab 150 mg (Part B) and 450 mg (Parts A and B) (Table 1 and Figure 1). In Part A, TNFα and IL-10 concentrations were reduced (nominal P< 0.05) with litifilimab 450 mg at W24 (Table 1); no strong evidence of this was seen in Part B. Change in IFNα concentration and change in total active joint count with litifilimab 450 mg in Part A were correlated (Spearman correlation estimates: PBO −0.15 [95% CI: −0.41, 0.16]; 450 mg 0.34 [0.07, 0.55]), as were changes in IFNα concentration and CLASI-A score (PBO −0.01 [−0.34, 0.31]; 450 mg 0.35 [0.10, 0.58]) at W24. A trend of greater median IFNα concentration reduction in W24 SRI-4 responders relative to non-responders was seen with litifilimab 450 mg. Confirmation of these findings in larger sample sizes is warranted. In Part B, the dose-response relationship was similar to that observed with the clinical responses (Figure 2) and percent change in CLASI-A score was correlated with percent change in IFNGS score in all treatment groups.
Conclusion: Litifilimab induced a rapid, substantial, and sustained reduction in 22-gene IFNGS scores and IFNα concentrations, and reductions in some biomarkers correlated with clinical responses. These results further support the mechanism of action for litifilimab and the role of Type I IFN and pDCs in SLE and CLE.5,6
1Furie R et al. N Engl J Med 2022;387:894–904
2Werth V et al. N Engl J Med 2022;387:321–331
3Werth V et al. J Invest Dermatol 2023;143(5 Supp):S101
4Furie R et al. Ann Rheum Dis 2023;82(1 Supp):1453–1454
5Fetter T et al. Front Med (Lausanne) 2022;9:915828
6Rönnblom L, Leonard D. Lupus Sci Med 2019;6:e000270
.jpg)
.jpg)
R. Furie: Biogen, 2, 5; V. Werth: Abbvie, 2, Amgen, 2, 5, AnaptysBio, 2, Argenx, 5, AstraZeneca, 2, Biogen, 2, 5, Bristol Myers Squibb, 2, Celgene, 2, 5, Corbus, 5, CSL Behring, 2, 5, EMD Serono, 2, Galderma, 2, Genentech/Roche PI, 5, Gilead, 2, 5, GSK, 2, Horizon Therapeutics, 5, Janssen, 2, Kyowa Kirin, 2, Lilly, 2, Merck, 2, Nektar, 2, Novartis, 2, Octapharma, 2, Pfizer, 2, 5, Regeneron, 5, Rome Therapeutics, 2, 5, Sanofi, 2, Ventus, 5, Viela, 2, 5, Xencor, 2; E. Milliman: Biogen, 3, 11; K. Ferber: Biogen, 3, 11; F. Casey: Biogen, 3, 11; R. Brown: Biogen, 3, 11; D. Raitcheva: Biogen, 3, 10, 11, Novartis, 3, 11; J. Zoghbi: Biogen, 3, 11; D. Graham: Biogen, 3, 11; G. Kong: Biogen, 3, 11; Y. Lahoud: Biogen, 3, 11; N. Franchimont: Alexion/AstraZeneca, 3, 11, Biogen, 3, 11, Nimbus Therapeutics, 3, 11, OMass Therapeutics, 4, 11; C. Barbey: Biogen, 3, 11.
Background/Purpose: Litifilimab is a humanized IgG1 mAb targeting BDCA2, a receptor expressed on plasmacytoid dendritic cells (pDCs), that negatively regulates the production of Type I IFN and proinflammatory chemokines and cytokines.1,2 In the Phase 2 LILAC study of litifilimab (NCT02847598), Part A (participants with SLE, active arthritis and rash) and Part B (participants with active CLE, with or without SLE) met their primary endpoints at Weeks (W) 24 and 16, respectively.1,2 We evaluated the effects of litifilimab on IFN gene signature (IFNGS) scores from whole blood samples, serum IFNα concentrations, and other serum cytokine concentrations in LILAC participants.3,4
Methods: Expression of 22-gene panel IFNGS scores and concentrations of IFNα and other cytokines were examined over time in the modified intent-to-treat population (Part A N=120, Part B N=132) with available data at baseline (BL) and ≥1 post-BL visit. Treatment effects were estimated using a mixed model repeated measures approach. In Part A, IFNα concentration changes over time were compared with W24 total active joint counts and CLASI-A scores (Spearman rank correlations) and SLE Responder Index (SRI-4) status. In Part B, Pearson correlations between percent changes in CLASI-A score and IFNGS score were estimated and the dose-response relationship was estimated using a previously described MCP-Mod method.2
Results: As too few participants were IFNGS-low at BL, only data for the IFNGS-high subgroups are described. Litifilimab induced rapid, substantial, and sustained reductions in 22-gene IFNGS scores and IFNα concentrations that were greater (nominal P< 0.05) vs placebo (PBO) with litifilimab 150 mg (Part B) and 450 mg (Parts A and B) (Table 1 and Figure 1). In Part A, TNFα and IL-10 concentrations were reduced (nominal P< 0.05) with litifilimab 450 mg at W24 (Table 1); no strong evidence of this was seen in Part B. Change in IFNα concentration and change in total active joint count with litifilimab 450 mg in Part A were correlated (Spearman correlation estimates: PBO −0.15 [95% CI: −0.41, 0.16]; 450 mg 0.34 [0.07, 0.55]), as were changes in IFNα concentration and CLASI-A score (PBO −0.01 [−0.34, 0.31]; 450 mg 0.35 [0.10, 0.58]) at W24. A trend of greater median IFNα concentration reduction in W24 SRI-4 responders relative to non-responders was seen with litifilimab 450 mg. Confirmation of these findings in larger sample sizes is warranted. In Part B, the dose-response relationship was similar to that observed with the clinical responses (Figure 2) and percent change in CLASI-A score was correlated with percent change in IFNGS score in all treatment groups.
Conclusion: Litifilimab induced a rapid, substantial, and sustained reduction in 22-gene IFNGS scores and IFNα concentrations, and reductions in some biomarkers correlated with clinical responses. These results further support the mechanism of action for litifilimab and the role of Type I IFN and pDCs in SLE and CLE.5,6
1Furie R et al. N Engl J Med 2022;387:894–904
2Werth V et al. N Engl J Med 2022;387:321–331
3Werth V et al. J Invest Dermatol 2023;143(5 Supp):S101
4Furie R et al. Ann Rheum Dis 2023;82(1 Supp):1453–1454
5Fetter T et al. Front Med (Lausanne) 2022;9:915828
6Rönnblom L, Leonard D. Lupus Sci Med 2019;6:e000270
.jpg)
Table 1. Primary efficacy endpoints and changes from BL in 22-gene panel IFNGS score and IFNα concentration for the IFNGS-high subgroup (modified intent-to-treat population). Data are LS means unless otherwise specified. Variations in n within each group at any timepoint are the result of missing samples and/or different analysis populations. *Assessed in participants who met the joint count inclusion criterion for Part A: ≥4 tender joints and ≥4 swollen joints according to a 28-joint assessment (≥4 swollen joints must have been in PIP, MCP, or wrist joints; participants were not required to have coexistent swelling and tenderness of individual joints);1 †total active joint count is the sum of the tender joint count and the swollen joint count; ‡ratio between the GM fold change from BL in the litifilimab arm and the GM fold change from BL in the placebo arm; values <1 favor litifilimab. BL, baseline; CI, confidence interval; CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity; GM, geometric mean; GMR, geometric mean ratio; IFNGS, interferon gene signature; LS, least squares; PBO, placebo
.jpg)
Figure 1. Change in IFNGS score and IFNα concentration over time in Part A (A and B) and Part B (C and D) of the Phase 2 LILAC study for the IFNGS-high subgroup. *At Week 24 (Part A), nominally significant (P<0.05) changes from BL in 22-gene panel IFNGS score and IFNα concentration were demonstrated for the litifilimab 450-mg group relative to placebo using GMRs; †at Week 16 (Part B), changes in IFNGS score and IFNα concentration were nominally significant (P<0.05) for the litifilimab 150- and 450-mg groups relative to placebo using GMRs. BL, baseline; CI, confidence interval; GMR, geometric mean ratio; IFNGS, interferon gene signature; LS, least squares

Figure 2. MCP-Mod analysis from Part B of the Phase 2 LILAC study for fold change in IFNGS score (A), fold change in IFNα concentration (B), and percent change in CLASI-A score (clinical response; C) at Week 16 for the IFNGS-high subgroup. Dose-response relationships are based on the MCP-Mod method. The MCP-Mod method uses a two-step process: a multiple comparison step to test for the presence of a significant dose-response relationship, in which placebo serves as a dose of 0 mg, and a modeling step to fit the best dose-response curve. Further details can be found in Werth VP, et al (2022).2 *CLASI-A data include participants with missing baseline biomarkers data. CI, confidence interval; CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity; IFNGS, interferon gene signature; MCP-Mod, multiple comparison procedure–modeling
R. Furie: Biogen, 2, 5; V. Werth: Abbvie, 2, Amgen, 2, 5, AnaptysBio, 2, Argenx, 5, AstraZeneca, 2, Biogen, 2, 5, Bristol Myers Squibb, 2, Celgene, 2, 5, Corbus, 5, CSL Behring, 2, 5, EMD Serono, 2, Galderma, 2, Genentech/Roche PI, 5, Gilead, 2, 5, GSK, 2, Horizon Therapeutics, 5, Janssen, 2, Kyowa Kirin, 2, Lilly, 2, Merck, 2, Nektar, 2, Novartis, 2, Octapharma, 2, Pfizer, 2, 5, Regeneron, 5, Rome Therapeutics, 2, 5, Sanofi, 2, Ventus, 5, Viela, 2, 5, Xencor, 2; E. Milliman: Biogen, 3, 11; K. Ferber: Biogen, 3, 11; F. Casey: Biogen, 3, 11; R. Brown: Biogen, 3, 11; D. Raitcheva: Biogen, 3, 10, 11, Novartis, 3, 11; J. Zoghbi: Biogen, 3, 11; D. Graham: Biogen, 3, 11; G. Kong: Biogen, 3, 11; Y. Lahoud: Biogen, 3, 11; N. Franchimont: Alexion/AstraZeneca, 3, 11, Biogen, 3, 11, Nimbus Therapeutics, 3, 11, OMass Therapeutics, 4, 11; C. Barbey: Biogen, 3, 11.



