Poster Session A
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (0283–0307) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
0298: Accuracy of the 2017 European Alliance of Associations for Rheumatology (EULAR)/American College of Rheumatology (ACR) Classification Criteria and Myositis-Specific Autoantibodies-Based Classification Criteria for Classifying Patients with Idiopathic Inflammatory Myopathy
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MD
Marta Domínguez, PhD
Sociedad Española de Reumatología
Madrid, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Tatiana Cobo1, Marta Domínguez2, Anna Pros3, Jose Luís Tandaipán4, Laura Nuño5, JULIA MARTINEZ BARRIO6, Vega Jovani7, Fredeswinda Romero8, Maria Esther Ruiz Lucea9, Eva Tomero Muriel10, Ernesto Trallero Araguás11, Javier Narvaez12, Jordi Camins Fàbregas13, ALBERTO MARIANO RUIZ ROMAN14, Jesus Loarce-Martos15, Annika Nack16, Esmeralda Delgado-Frías17, Francisca Sivera18, carolina Merino19, Antonio Juan Mas20, Alejandro Gómez Gómez1, Jose-Maria Pego-Reigosa21, Maria Martin-Lopez22, Jesús Alejandro Valero23, Carmen carrasco-Cubero24, Mercedes Freire González25, Iñigo Rúa-Figueroa26, Nuria Lozano Rivas27, Julio David Suarez Cuba28, Ana Isabel Turrión Nieves29, María Ángeles Puche Larrubia30 and Patricia Alcocer Amores31, 1Department of Rheumatology, Hospital Universitario Infanta Sofía, Universidad Europea, Madrid, Spain, 2Sociedad Española de Reumatología, Madrid, Spain, 3Department of Rheumatology, Hospital del Mar, Barcelona, Spain, 4Department of Rheumatology, Hospital Universitari de la Santa Creu i Sant Pau, Barcelona, Spain, 5Hospital Universitario La Paz - IdiPAZ, Madrid, Spain, 6Rheumatology, Gregorio Marañon University Hospital, Madrid, Spain, 7Department of Rheumatology, Hospital General Universitario Dr. Balmis, Alicante, Spain, 8IIS-HU Fundación Jiménez Díaz, Madrid, Spain, 9Basurto University Hospital, Bilbao, Spain, 10Rheumatology, Hospital La Princesa, Madrid, Spain, 11Department of Rheumatology, Hospital Universitario Vall d'Hebron, Barcelona, Spain, 12Hospital Universitario de Bellvitge, Barcelona, Spain, 13Department of Rheumatology, Hospital General de Granollers, Barcelona, Spain, 14Department of Rheumatology, Hospital Universitario Virgen del Rocío, Sevilla, Spain, 15Ramón y Cajal University Hospital, Madrid, Spain, 16Department of Rheumatology, Hospital Universitari Germans Trias i Pujol, Barcelona, Spain, 17Department of Rheumatology, Hospital Universitario de Canarias, Tenerife, Spain, 18Department of Rheumatology, Hospital General Universitario de Elda, Alicante, Spain, 19Rheumatology Department Hospital Universitario Puerta de Hierro, Majadahonda (Madrid), Spain, 20Hospital Universitario Son Llàtzer, Mallorca, Spain, 21Rheumatology, Hospital do Meixoeiro, Vigo, Spain, 22Hospital 12 de Octubre, Madrid, Spain, 23Department of Rheumatology, Hospital Universitario Donostia, San Sebastián, Spain, 24Department of Rheumatology, Hospital Universitario de Badajoz, Badajoz, Spain, 25Rheumatology department, Complexo Hospitalario Universitario A Coruña (CHUAC). Instituto de Investigación Biomédica A Coruña (INIBIC), A Coruña, Spain, 26Rheumatology, Hospital de Gran Canaria Doctor Negrin, Las Palmas de Gran Canaria, Spain, 27Department of Rheumatology, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain, 28Department of Rheumatology, Hospital Universitario Príncipe de Asturias, Madrid, Spain, 29Department of Rheumatology, Hospital Complejo Asistencial Universitario de Salamanca, Salamanca, Spain, 30Department of Rheumatology, Reina Sofia University Hospital, Cordoba, Spain, 31Department of Rheumatology, Hospital Universitario HM Sanchinarro, Madrid, Spain
Background/Purpose: Limitations of the 2017 EULAR/ACR classification criteria have been suggested for classifying patients with idiopathic inflammatory myopathies (IIMs) and myositis-specific antibodies (MSAs). On this point, Casal-Dominguez et al recently developed a set of MSAs-based classification criteria that demonstrated perfect sensitivity and specificity (1).
The objective of this study was to determine whether the EULAR/ACR classification criteria and the MSAs-based classification criteria appropriately classify patients with IIMs, differentiating between incident and prevalent cases
Methods: Multicenter cross-sectional study of a cohort of patients included in the Spanish Registry of patients with IIM (Myo-Spain) (2). Patients were classified as incident group (time between diagnosis and study initiation ≤12 months) or prevalent group ( >12 months). The accuracy of the classification criteria according of the presence of different MSAs was described. Differences between both groups were tested by Chi-square test. The sensitivity and specificity of the MSAs-based classification criteria was determined. The percent of agreement and the Cohen's Kappa coefficient was used to measured correlations between the EULAR/ACR criteria and the MSAs-based criteria
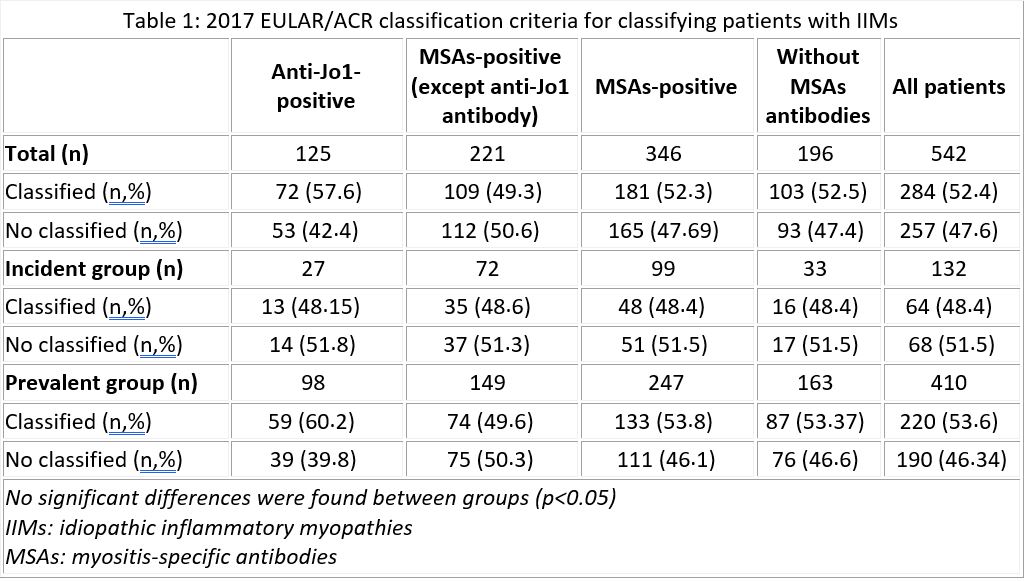
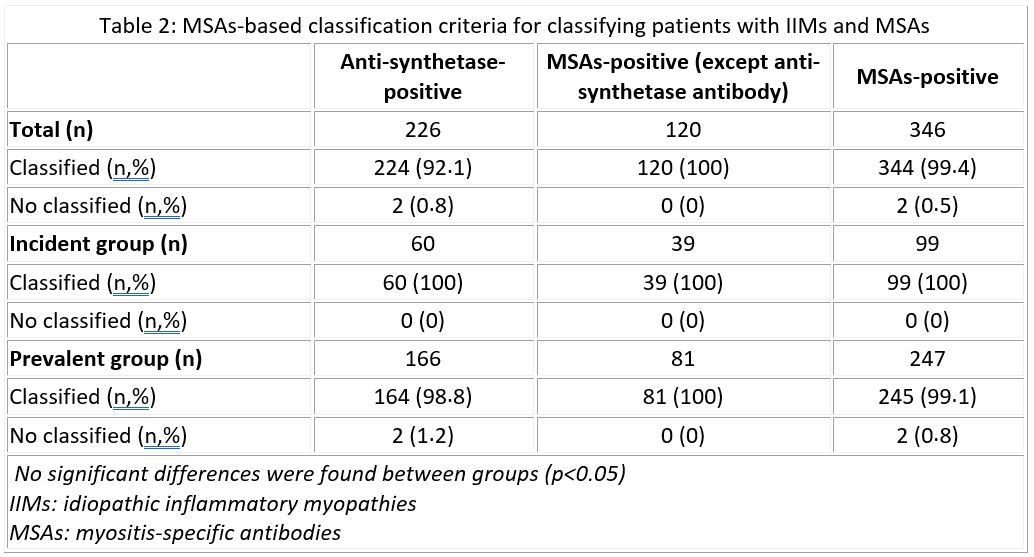
Results: We included 542 patients with IIM diagnosis, 132 (24.4%) and 410 (75.6%) patients in the incident and prevalent group, respectively. In the overall sample, the diagnosis could be classified with the EULAR/ACR criteria in 284 patients (52.4%) and with the MSAs-based criteria in 344 patients (99.4%). Differentiating by MSAs type, patients were successfully classified by EULAR/ACR criteria as follows: 57.6 % of anti-Jo1-positive patients; 49.3% of MSAs-positive myositis patients (except anti-Jo1 antibody); 52.3% of MSAs-positive myositis patients; 52.5% of patients without MSAs antibodies (table 1). No significant differences were found between the two groups (p >0,05). Regarding MSAs-based classification criteria, patients were successfully classified as follows: 92.1% of anti-synthetase-positive patients and 100% of MSAs-positive (except anti-synthetase antibody) patients (table 2). No significant differences were found between the two groups (p >0,05).The sensitivity and specificity of the MSAs-based criteria were 100% in the incident group and 99.2% and 100%, respectively, in the prevalent group. The percentage of agreement between the EULAR/ACR criteria and the MSAs-based criteria for IIMs was 49.2% in the incident group and 50.9% in the prevalent group. This value was 48.1% and 60.2% in anti-Jo1-positive subgroup (Cohen's Kappa=0).
Conclusion: The degree of accuracy of the EULAR/ACR criteria for classifying patients with IIM diagnosis was lo, however, the MSAs-based criteria showed excellent diagnostic accuracy. No agreement was found between both classification criteria. Therefore, it seems necessary to review the classification criteria for MII, in which, in addition to including all MSAs, the difficulty in classifying patients without MSAs should be considered.
(1) Casal-Dominguez M et al. Arthritis Rheumatol 2022. (2) Cobo-Ibáñez T et al. Reumatol Clin. 2022.
T. Cobo: None; M. Domínguez: None; A. Pros: None; J. Tandaipán: None; L. Nuño: None; J. MARTINEZ BARRIO: None; V. Jovani: None; F. Romero: None; M. Ruiz Lucea: None; E. Tomero Muriel: None; E. Trallero Araguás: None; J. Narvaez: None; J. Camins Fàbregas: None; A. RUIZ ROMAN: None; J. Loarce-Martos: Boehringer-Ingelheim, 6, Bristol-Myers Squibb(BMS), 6, Galapagos, 6; A. Nack: None; E. Delgado-Frías: None; F. Sivera: AbbVie/Abbott, 1, AstraZeneca, 5, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 5, 6, GlaxoSmithKlein(GSK), 6, Novartis, 5, Pfizer, 1, Roche, 5, UCB, 6; c. Merino: None; A. Mas: None; A. Gómez Gómez: Amgen, 6, Janssen, 6, Novartis, 5, Pfizer, 5, UCB, 5, 5; J. Pego-Reigosa: None; M. Martin-Lopez: None; J. Valero: None; C. carrasco-Cubero: None; M. Freire González: None; I. Rúa-Figueroa: AstraZeneca, 5, GSK, 1, 6; N. Lozano Rivas: None; J. Suarez Cuba: None; A. Turrión Nieves: None; M. Puche Larrubia: None; P. Alcocer Amores: None.
Background/Purpose: Limitations of the 2017 EULAR/ACR classification criteria have been suggested for classifying patients with idiopathic inflammatory myopathies (IIMs) and myositis-specific antibodies (MSAs). On this point, Casal-Dominguez et al recently developed a set of MSAs-based classification criteria that demonstrated perfect sensitivity and specificity (1).
The objective of this study was to determine whether the EULAR/ACR classification criteria and the MSAs-based classification criteria appropriately classify patients with IIMs, differentiating between incident and prevalent cases
Methods: Multicenter cross-sectional study of a cohort of patients included in the Spanish Registry of patients with IIM (Myo-Spain) (2). Patients were classified as incident group (time between diagnosis and study initiation ≤12 months) or prevalent group ( >12 months). The accuracy of the classification criteria according of the presence of different MSAs was described. Differences between both groups were tested by Chi-square test. The sensitivity and specificity of the MSAs-based classification criteria was determined. The percent of agreement and the Cohen's Kappa coefficient was used to measured correlations between the EULAR/ACR criteria and the MSAs-based criteria
Results: We included 542 patients with IIM diagnosis, 132 (24.4%) and 410 (75.6%) patients in the incident and prevalent group, respectively. In the overall sample, the diagnosis could be classified with the EULAR/ACR criteria in 284 patients (52.4%) and with the MSAs-based criteria in 344 patients (99.4%). Differentiating by MSAs type, patients were successfully classified by EULAR/ACR criteria as follows: 57.6 % of anti-Jo1-positive patients; 49.3% of MSAs-positive myositis patients (except anti-Jo1 antibody); 52.3% of MSAs-positive myositis patients; 52.5% of patients without MSAs antibodies (table 1). No significant differences were found between the two groups (p >0,05). Regarding MSAs-based classification criteria, patients were successfully classified as follows: 92.1% of anti-synthetase-positive patients and 100% of MSAs-positive (except anti-synthetase antibody) patients (table 2). No significant differences were found between the two groups (p >0,05).The sensitivity and specificity of the MSAs-based criteria were 100% in the incident group and 99.2% and 100%, respectively, in the prevalent group. The percentage of agreement between the EULAR/ACR criteria and the MSAs-based criteria for IIMs was 49.2% in the incident group and 50.9% in the prevalent group. This value was 48.1% and 60.2% in anti-Jo1-positive subgroup (Cohen's Kappa=0).
Conclusion: The degree of accuracy of the EULAR/ACR criteria for classifying patients with IIM diagnosis was lo, however, the MSAs-based criteria showed excellent diagnostic accuracy. No agreement was found between both classification criteria. Therefore, it seems necessary to review the classification criteria for MII, in which, in addition to including all MSAs, the difficulty in classifying patients without MSAs should be considered.
(1) Casal-Dominguez M et al. Arthritis Rheumatol 2022. (2) Cobo-Ibáñez T et al. Reumatol Clin. 2022.

2017 EULAR/ACR classification criteria for classifying patients with IIMs

MSAs-based classification criteria for classifying patients with IIMs and MSAs
T. Cobo: None; M. Domínguez: None; A. Pros: None; J. Tandaipán: None; L. Nuño: None; J. MARTINEZ BARRIO: None; V. Jovani: None; F. Romero: None; M. Ruiz Lucea: None; E. Tomero Muriel: None; E. Trallero Araguás: None; J. Narvaez: None; J. Camins Fàbregas: None; A. RUIZ ROMAN: None; J. Loarce-Martos: Boehringer-Ingelheim, 6, Bristol-Myers Squibb(BMS), 6, Galapagos, 6; A. Nack: None; E. Delgado-Frías: None; F. Sivera: AbbVie/Abbott, 1, AstraZeneca, 5, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 5, 6, GlaxoSmithKlein(GSK), 6, Novartis, 5, Pfizer, 1, Roche, 5, UCB, 6; c. Merino: None; A. Mas: None; A. Gómez Gómez: Amgen, 6, Janssen, 6, Novartis, 5, Pfizer, 5, UCB, 5, 5; J. Pego-Reigosa: None; M. Martin-Lopez: None; J. Valero: None; C. carrasco-Cubero: None; M. Freire González: None; I. Rúa-Figueroa: AstraZeneca, 5, GSK, 1, 6; N. Lozano Rivas: None; J. Suarez Cuba: None; A. Turrión Nieves: None; M. Puche Larrubia: None; P. Alcocer Amores: None.



