Poster Session A
Osteoarthritis (OA) and related disorders
Session: (0308–0324) Osteoarthritis – Clinical Poster I
0323: A Randomized, Double-blind, Placebo-controlled, Repeat Injection, 52-Week Study to Evaluate the Efficacy and Safety of an Intra-articular Injection (IAI) of CNTX-4975-05 (CNTX) in Subjects with Chronic, Moderate-to-severe Osteoarthritis Knee Pain (MSOAKP) - Study 304
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- RS
Randall Stevens, MD
Centrexion Therapeutics Corp.
Rockport, MA, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
James Connolly, James N Campbell, Randall Stevens and Colleen Newman, Centrexion Therapeutics Corporation, Boston, MA
Background/Purpose: 30+ million US adults have OAKP. After years of using NSAIDs, analgesics, surgery and IAI, many patients remain with MSOAKP. CNTX is a 1 mg dose of capsaicin for IAI to reduce MSOAKP.
Methods: Efficacy and safety of a two IAI doses of placebo or 1 mg CNTX (Day 1 and at Week 26; ratio capsaicin/placebo 3:2; blinded randomization) were assessed in subjects with chronic, MSOAKP. Subjects were allowed specific MSOAKP concomitant and rescue medications during the study.
Pain was assessed using: a numeric pain rating scale (NPRS [0 – 10; 0 = no pain, 10 worst pain possible]). Subjects had Kellgren-Lawrence (KL) grades 2-4 (radiograph; 0=normal, 4=severe), and met knee OA (KOA) diagnostic criteria. Subjects were blindly randomized. The primary endpoint (Week 12) was the Western Ontario and McMaster Universities Osteoarthritis Index Subscale A (pain). Subjects were also assessed on WOMAC B (stiffness), WOMAC C (function), Numeric Pain Rating Scale (NPRS; 0-10), and Patient Global Impression of Change (PGIC; 0-7).
Key safety included physical exams, vital signs, ECGs, labs and bilateral fixed flexion knee radiographs at screening, and Week 52.
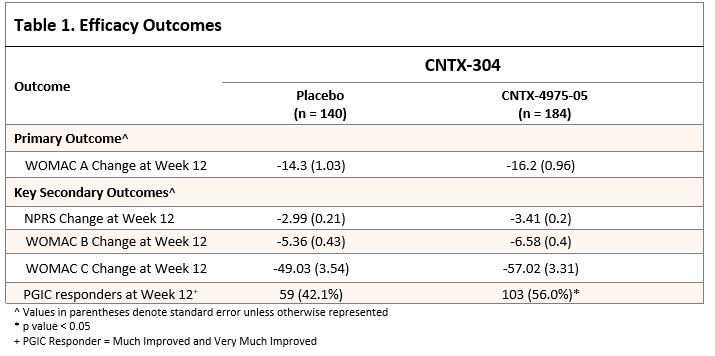
Results: 325 subjects received 1 dose of study drug (140 placebo, 184 CNTX); 243 randomized subjects completed out to Week 52; 89 subjects discontinued (DC) early. DC reasons were subject withdrawal (6.9%), lack of efficacy (6.3%) and other (5.1%). 15 (4.5%) subjects DC prior to Week 12. Mean age 62.8 years (range = 40-84), 61.4% female, 71.3% - not Hispanic or Latino; 70.4% Caucasian and 26.5% Back/African American.
Mean index knee baseline NPRS was 7.0, WOMAC A (0-50 scale; 0 = severe pain) 31.5; WOMAC B (0-20 scale) and WOMAC C (0-170) was 13.2 and 108.8, respectively.
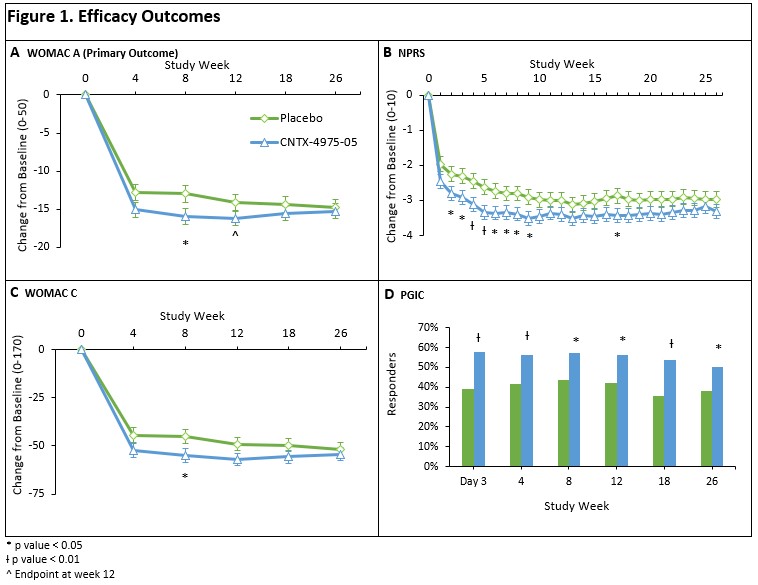
Least-squares (LS) mean reduction was greater for CNTX than placebo (−16.20 vs -14.13, respectively; p = 0.0833). The WOMAC B / C, and NPRS were numerically better at nearly all visits through Week 26, and usually better to Week 52, for CNTX relative to placebo. Also, a greater proportion of CNTX subjects were considered PGIC responders at Week 12 and beyond – After the second IAI at Week 26, both pain and function improvements were seen in CNTX vs placebo, up to Week 52. (Table 1 and Figure 1).
There was more transient post injection pain on Day 1 in the CNTX group than placebo. Safety was acceptable out to Week 52 with knee radiographs showing no difference in KOA progression between placebo and CNTX, and no rapidly progressive osteoarthritis.
Conclusion: This study did not meet the primary endpoint. However, the numeric data suggested greater analgesic efficacy through Week 26 in CNTX compared to placebo. CNTX safety was acceptable. The NPRS difference was significant at several timepoints.
J. Connolly: Centrexion Therapeutics Corporation, 3; J. Campbell: None; R. Stevens: Centrexion Therapeutics Corporation, 3; C. Newman: Centrexion Therapeutics Corp, 3.
Background/Purpose: 30+ million US adults have OAKP. After years of using NSAIDs, analgesics, surgery and IAI, many patients remain with MSOAKP. CNTX is a 1 mg dose of capsaicin for IAI to reduce MSOAKP.
Methods: Efficacy and safety of a two IAI doses of placebo or 1 mg CNTX (Day 1 and at Week 26; ratio capsaicin/placebo 3:2; blinded randomization) were assessed in subjects with chronic, MSOAKP. Subjects were allowed specific MSOAKP concomitant and rescue medications during the study.
Pain was assessed using: a numeric pain rating scale (NPRS [0 – 10; 0 = no pain, 10 worst pain possible]). Subjects had Kellgren-Lawrence (KL) grades 2-4 (radiograph; 0=normal, 4=severe), and met knee OA (KOA) diagnostic criteria. Subjects were blindly randomized. The primary endpoint (Week 12) was the Western Ontario and McMaster Universities Osteoarthritis Index Subscale A (pain). Subjects were also assessed on WOMAC B (stiffness), WOMAC C (function), Numeric Pain Rating Scale (NPRS; 0-10), and Patient Global Impression of Change (PGIC; 0-7).
Key safety included physical exams, vital signs, ECGs, labs and bilateral fixed flexion knee radiographs at screening, and Week 52.
Results: 325 subjects received 1 dose of study drug (140 placebo, 184 CNTX); 243 randomized subjects completed out to Week 52; 89 subjects discontinued (DC) early. DC reasons were subject withdrawal (6.9%), lack of efficacy (6.3%) and other (5.1%). 15 (4.5%) subjects DC prior to Week 12. Mean age 62.8 years (range = 40-84), 61.4% female, 71.3% - not Hispanic or Latino; 70.4% Caucasian and 26.5% Back/African American.
Mean index knee baseline NPRS was 7.0, WOMAC A (0-50 scale; 0 = severe pain) 31.5; WOMAC B (0-20 scale) and WOMAC C (0-170) was 13.2 and 108.8, respectively.
Least-squares (LS) mean reduction was greater for CNTX than placebo (−16.20 vs -14.13, respectively; p = 0.0833). The WOMAC B / C, and NPRS were numerically better at nearly all visits through Week 26, and usually better to Week 52, for CNTX relative to placebo. Also, a greater proportion of CNTX subjects were considered PGIC responders at Week 12 and beyond – After the second IAI at Week 26, both pain and function improvements were seen in CNTX vs placebo, up to Week 52. (Table 1 and Figure 1).
There was more transient post injection pain on Day 1 in the CNTX group than placebo. Safety was acceptable out to Week 52 with knee radiographs showing no difference in KOA progression between placebo and CNTX, and no rapidly progressive osteoarthritis.
Conclusion: This study did not meet the primary endpoint. However, the numeric data suggested greater analgesic efficacy through Week 26 in CNTX compared to placebo. CNTX safety was acceptable. The NPRS difference was significant at several timepoints.


J. Connolly: Centrexion Therapeutics Corporation, 3; J. Campbell: None; R. Stevens: Centrexion Therapeutics Corporation, 3; C. Newman: Centrexion Therapeutics Corp, 3.



