Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0582–0608) SLE – Treatment Poster I
0597: Efficacy of Anifrolumab in Systemic Lupus Erythematosus by Overall and Organ-Specific SLEDAI-2K Improvements: Results from the Randomized, Placebo-Controlled Phase 3 Long-Term Extension Study
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Richard Furie, MD
Chief of the Division of Rheumatology
Northwell Health
Manhasset, NY, NY, United StatesDisclosure(s): Biogen: Consultant (Ongoing), Grant/Research Support (Ongoing)
Abstract Poster Presenter(s)
Richard A. Furie1, Kenneth Kalunian2, Eric Morand3, Ian Bruce4, Susan Manzi5, Yoshiya Tanaka6, Kevin Withrop7, Ihor Hupka8, Micki Hultquist9, Raj Tummala9, Gabriel Abreu10, Catharina Lindholm10 and Hussein Al-Mossawi11, 1Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Great Neck, NY, 2University of California San Diego, La Jolla, CA, 3Monash University, Centre for Inflammatory Diseases, Melbourne, Australia, 4University of Manchester, Manchester, United Kingdom, 5Lupus Center of Excellence, Autoimmunity Institute, Allegheny Health Network, Pittsburgh, PA, 6University of Occupational and Environmental Health, Kitakyushu, Japan, 7Oregon Health & Science University, Schools of Medicine and Public Health,, Portland, OR, 8BioPharmaceuticals R&D, AstraZeneca, Warsaw, Poland, 9BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, MD, 10BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden, 11BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom
Background/Purpose: SLE is a systemic autoimmune disease requiring long-term treatment. In this placebo-controlled phase 3 TULIP long-term extension (LTE) study,1 the impact of anifrolumab in addition to standard therapy on total and organ-specific disease activity was assessed over 4 years in patients with moderate to severe SLE.
Methods: TULIP-LTE (NCT02794285) was the first randomized, placebo-controlled, double-blind, 3-year extension study in SLE. Patients with SLE (1997 ACR criteria) on standard therapy who completed a TULIP trial through the 52-week treatment period could enroll in the LTE. Treatment groups used in this analysis were patients randomized to receive intravenous anifrolumab 300 mg or placebo every 4 weeks in TULIP-1/-2 who continued the same treatment (or would have continued if not discontinued during TULIP-1/-2) in the LTE (anifrolumab 300 mg: n=358; placebo: n=178). Proportions of patients with a ≥4-point reduction in total SLEDAI-2K score from Week 0 (baseline) to Week 208 were compared between treatment groups using summary statistics. Proportions of patients with a SLEDAI-2K improvement (patients with improvement [n1] / patients with baseline involvement and available data at the timepoint [n2]) were further analyzed by organ domain (mucocutaneous, musculoskeletal, immunological, hematological/fever) during the TULIP and LTE periods; SLEDAI-2K improvement was defined as a lower organ domain score than at baseline.
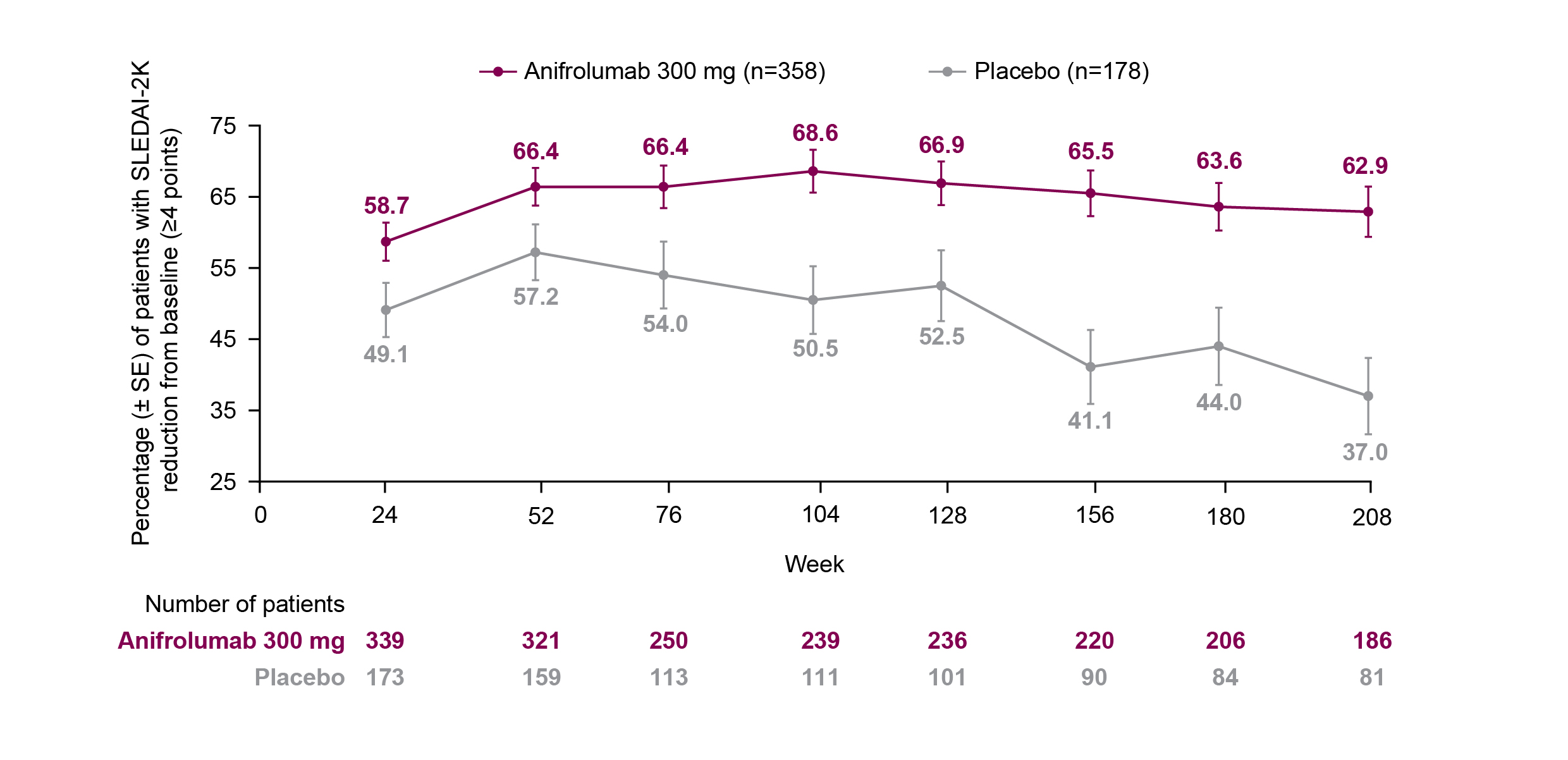
Results: The proportion of patients who achieved a ≥4-point reduction in SLEDAI-2K score from baseline was greater with anifrolumab vs placebo from Week 52 (66.4%, [95% confidence interval: 60.9–71.5] vs 57.2% [49.2–65.0]) to Week 208 (62.9% [55.5–69.9] vs 37.0% [26.6–48.5]), and the difference between the treatment groups increased over time (Figure). Baseline organ involvement was similar between treatment groups in the mucocutaneous and musculoskeletal domains (anifrolumab 300 mg vs placebo: 96.6% vs 95.5% and 93.0% vs 93.8%, respectively). Immunological and hematological/fever baseline involvement were higher in the anifrolumab group compared with placebo (65.6% vs 58.4% and 15.4 vs 9.0%, respectively). Mucocutaneous, musculoskeletal, immunological, and hematological/fever SLEDAI-2K improvements generally occurred more frequently in the anifrolumab group compared with placebo from Week 52 (73.5% [n1/n2: 227/309] vs 60.9% [92/151]; 67.9% [201/296] vs 64.9% [96/148]; 28.8% [61/212] vs 17.2% [16/93]; and 81.6% [40/49] vs 80.0% [12/15]) up to Week 208 (79.8% [142/178] vs 58.7% [44/75]; 71.8% [122/170] vs 56.0% [42/75]; 32.1% [36/112] vs 14.6% [6/41]; and 72.4% [21/29] vs 42.9% [3/7]). By the end of the LTE period, many patients had missing data in the placebo group, due to lack of organ involvement and/or no available data at the timepoint.
Conclusion: Compared with placebo, anifrolumab was associated with long-term, sustained ≥4-point reductions in SLEDAI-2K over the 4-year TULIP and LTE period and, despite the higher drop-out rates in the placebo group, greater improvements were seen in disease activity for individual organ domains. References: 1. Kalunian KC, et al. Arthritis Rheumatol. 2023;75:253–65.
R. Furie: AstraZeneca, 2, 5, 6; K. Kalunian: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Biogen, 2, Bristol-Myers Squibb, 2, Eli Lilly, 2, Genentech/Roche, 2, GlaxoSmithKline, 2, Janssen, 2, Pfizer, 2; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; I. Bruce: AstraZeneca, 1, 2, 5, 6, Aurinia, 2, GSK, 1, 2, 5, 6, Janssen, 5, 6, Lilly, 1, UCB, 6; S. Manzi: AbbVie, 5, Allegheny Singer Research Institute, 10, AstraZeneca, 2, 5, Exagen Diagnostics, Inc, 2, 9, 10, GSK, 2, 5, Lilly, 2, Lupus Foundation of America, 4, Novartis, 2, UCB Advisory Board, 2, Univiersity of Pittsburgh, 10; Y. Tanaka: AbbVie, 6, AstraZeneca, 6, BMS, 6, Boehringer-Ingelheim, 6, Chugai, 5, 6, Eisai, 5, 6, Eli Lilly, 6, Gilead, 6, GSK, 6, Mitsubishi-Tanabe, 5, Pfizer, 6, Taiho, 6, Taisho, 5, 6; K. Withrop: AbbVie, 2, AstraZeneca, 2, BMS, 2, 5, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Novartis, 2, Pfizer, 2, 5, Regeneron, 2, Roche, 2, Sanofi, 2, UCB, 2; I. Hupka: None; M. Hultquist: AstraZeneca, 3, 11; R. Tummala: AstraZeneca, 3; G. Abreu: AstraZeneca, 3; C. Lindholm: AstraZeneca, 3; H. Al-Mossawi: AstraZeneca, 3.
Background/Purpose: SLE is a systemic autoimmune disease requiring long-term treatment. In this placebo-controlled phase 3 TULIP long-term extension (LTE) study,1 the impact of anifrolumab in addition to standard therapy on total and organ-specific disease activity was assessed over 4 years in patients with moderate to severe SLE.
Methods: TULIP-LTE (NCT02794285) was the first randomized, placebo-controlled, double-blind, 3-year extension study in SLE. Patients with SLE (1997 ACR criteria) on standard therapy who completed a TULIP trial through the 52-week treatment period could enroll in the LTE. Treatment groups used in this analysis were patients randomized to receive intravenous anifrolumab 300 mg or placebo every 4 weeks in TULIP-1/-2 who continued the same treatment (or would have continued if not discontinued during TULIP-1/-2) in the LTE (anifrolumab 300 mg: n=358; placebo: n=178). Proportions of patients with a ≥4-point reduction in total SLEDAI-2K score from Week 0 (baseline) to Week 208 were compared between treatment groups using summary statistics. Proportions of patients with a SLEDAI-2K improvement (patients with improvement [n1] / patients with baseline involvement and available data at the timepoint [n2]) were further analyzed by organ domain (mucocutaneous, musculoskeletal, immunological, hematological/fever) during the TULIP and LTE periods; SLEDAI-2K improvement was defined as a lower organ domain score than at baseline.
Results: The proportion of patients who achieved a ≥4-point reduction in SLEDAI-2K score from baseline was greater with anifrolumab vs placebo from Week 52 (66.4%, [95% confidence interval: 60.9–71.5] vs 57.2% [49.2–65.0]) to Week 208 (62.9% [55.5–69.9] vs 37.0% [26.6–48.5]), and the difference between the treatment groups increased over time (Figure). Baseline organ involvement was similar between treatment groups in the mucocutaneous and musculoskeletal domains (anifrolumab 300 mg vs placebo: 96.6% vs 95.5% and 93.0% vs 93.8%, respectively). Immunological and hematological/fever baseline involvement were higher in the anifrolumab group compared with placebo (65.6% vs 58.4% and 15.4 vs 9.0%, respectively). Mucocutaneous, musculoskeletal, immunological, and hematological/fever SLEDAI-2K improvements generally occurred more frequently in the anifrolumab group compared with placebo from Week 52 (73.5% [n1/n2: 227/309] vs 60.9% [92/151]; 67.9% [201/296] vs 64.9% [96/148]; 28.8% [61/212] vs 17.2% [16/93]; and 81.6% [40/49] vs 80.0% [12/15]) up to Week 208 (79.8% [142/178] vs 58.7% [44/75]; 71.8% [122/170] vs 56.0% [42/75]; 32.1% [36/112] vs 14.6% [6/41]; and 72.4% [21/29] vs 42.9% [3/7]). By the end of the LTE period, many patients had missing data in the placebo group, due to lack of organ involvement and/or no available data at the timepoint.
Conclusion: Compared with placebo, anifrolumab was associated with long-term, sustained ≥4-point reductions in SLEDAI-2K over the 4-year TULIP and LTE period and, despite the higher drop-out rates in the placebo group, greater improvements were seen in disease activity for individual organ domains. References: 1. Kalunian KC, et al. Arthritis Rheumatol. 2023;75:253–65.

Figure. Percentage (± SE) of Patients With a SLEDAI-2K ≥4-point Reduction From Baseline* During the TULIP and LTE Period.
LTE, long-term extension; SE, standard error; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE Disease Activity Index 2000.
*Analysis includes patients with a SLEDAI-2K score ≥4 at TULIP baseline (Week 0) and data with discontinuation due to worsening/lack of efficacy non-improvement imputation.
LTE, long-term extension; SE, standard error; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE Disease Activity Index 2000.
*Analysis includes patients with a SLEDAI-2K score ≥4 at TULIP baseline (Week 0) and data with discontinuation due to worsening/lack of efficacy non-improvement imputation.
R. Furie: AstraZeneca, 2, 5, 6; K. Kalunian: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Biogen, 2, Bristol-Myers Squibb, 2, Eli Lilly, 2, Genentech/Roche, 2, GlaxoSmithKline, 2, Janssen, 2, Pfizer, 2; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; I. Bruce: AstraZeneca, 1, 2, 5, 6, Aurinia, 2, GSK, 1, 2, 5, 6, Janssen, 5, 6, Lilly, 1, UCB, 6; S. Manzi: AbbVie, 5, Allegheny Singer Research Institute, 10, AstraZeneca, 2, 5, Exagen Diagnostics, Inc, 2, 9, 10, GSK, 2, 5, Lilly, 2, Lupus Foundation of America, 4, Novartis, 2, UCB Advisory Board, 2, Univiersity of Pittsburgh, 10; Y. Tanaka: AbbVie, 6, AstraZeneca, 6, BMS, 6, Boehringer-Ingelheim, 6, Chugai, 5, 6, Eisai, 5, 6, Eli Lilly, 6, Gilead, 6, GSK, 6, Mitsubishi-Tanabe, 5, Pfizer, 6, Taiho, 6, Taisho, 5, 6; K. Withrop: AbbVie, 2, AstraZeneca, 2, BMS, 2, 5, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Novartis, 2, Pfizer, 2, 5, Regeneron, 2, Roche, 2, Sanofi, 2, UCB, 2; I. Hupka: None; M. Hultquist: AstraZeneca, 3, 11; R. Tummala: AstraZeneca, 3; G. Abreu: AstraZeneca, 3; C. Lindholm: AstraZeneca, 3; H. Al-Mossawi: AstraZeneca, 3.



