Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0543–0581) SLE – Diagnosis, Manifestations, & Outcomes Poster I
0558: Elevated Serum Levels of S100A8/A9 Discriminate Systemic Lupus Erythematosus Patients with Cognitive Impairment from Patients Without Impairment
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- CM
Carolina Munoz-Grajales, MD, PhD (she/her/hers)
UHN/TWH
Toronto, ON, CanadaDisclosure information not submitted.
Abstract Poster Presenter(s)
Carolina Munoz-Grajales1, Michelle Barraclough2, Juan Pablo Diaz Martinez3, Jiandong Su2, Kathleen Bingham2, Mahta Kakvan4, Roberta Kretzmann5, Carmela Tartaglia6, Lesley Ruttan7, May Choi8, Simone Appenzeller9, Sherief Marzouk5, Dennisse Bonilla2, Patti Katz10, Dorcas Beaton11, Robin Green5, Joan Wither2 and Zahi Touma5, 1UHN/TWH, Toronto, ON, Canada, 2University Health Network, Toronto, ON, Canada, 3Schroeder Arthritis Institute, University Health Network, Toronto, ON, Canada, 4University Health Network, University of Toronto, Toronto, ON, Canada, 5University of Toronto, Toronto, ON, Canada, 6Krembil Brain Institute, University Health Network, University of Toronto, Toronto, ON, Canada, 7University Health Network, Toronto Rehabilitation Institute, Toronto, ON, Canada, 8University of Calgary, Calgary, AB, Canada, 9UNICAMP, Campinas, Brazil, 10University of California San Francisco, San Rafael, CA, 11Institute for Work & Health, Toronto, ON, Canada
Background/Purpose: Cognitive impairment (CI) is one of the most common manifestations of Neuropsychiatric Systemic Lupus Erythematosus (NPSLE). Studies have reported that SLE patients with different NPSLE syndromes have alterations in the cerebrospinal fluid or their serum levels of various cytokines and proteases (analytes) that can lead to neuroinflammation. However, studies focused on analytes in CI are scarce. In this study, we investigated the ability of various serum analytes to discriminate SLE patients with CI from those without impairment.
Methods: Two hundred ninety individuals 18-65 years old who met the 2019 EULAR/ACR classification criteria for SLE were included. Cognitive ability was measured by psychometrists utilizing the 1-hr ACR-Neuropsychological Battery (ACR-NB), and the scores were standardized by age and gender to operationally classify patients' performance into CI (z-score of ≤-1.5 in two or more domains) and non-CI. At the time of the neuropsychological assessment patients provided blood samples. The serum levels of nine analytes (IL-6, IL-10, IFN-γ, MMP-9, NGAL, S100A8/A9, S100B, TNF-α, and TWEAK) were determined using ELISA. The data were randomly partitioned into a training (70%) and a test (30%) sets. A predictive regression model was performed to evaluate the measured analytes' ability to discriminate SLE patients with CI from patients without impairment. The optimal cut-off values to discern between CI and non-CI for each analyte were obtained by Youden's index, and their sensitivity (Sn), specificity (Sp), and predictive values were calculated. For patients that had completed cognitive assessment at 6 and 12 months (n=125, 43%), pairwise comparisons of the S100A8/A9 serum levels between time points were performed.
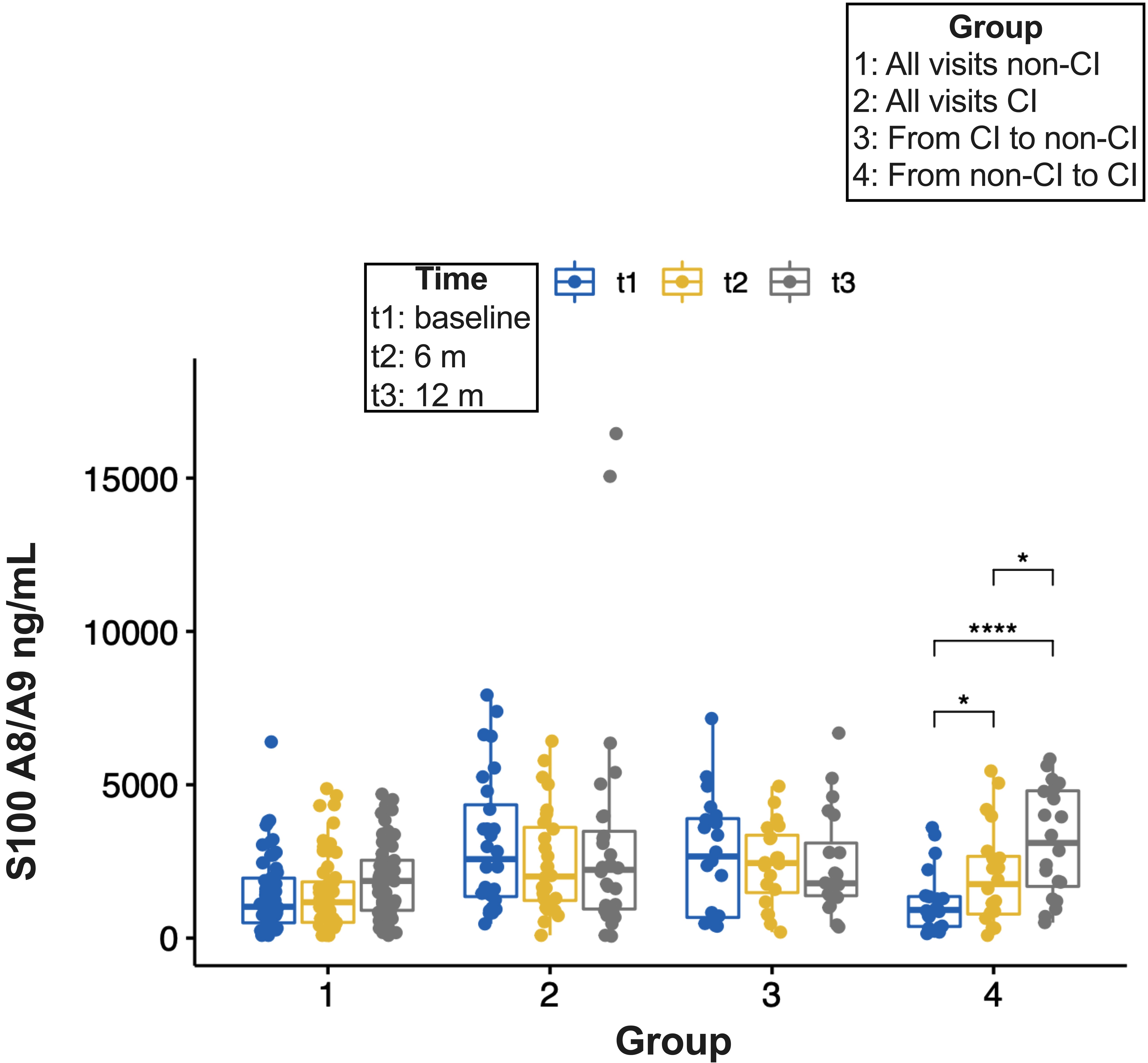
Results: Of 290 patients, 40% had CI (n=116). Overall, no differences in demographic or clinical characteristics were observed between patients with and without CI. S100A8/A9 had the highest AUC (0.79, 95% CI: 0.66-0.90) (Table 1) and displayed the greatest discriminative ability (Sp 86% and PPV 82%) (Table 2) to differentiate between patients with and without CI. Compared to the predictive ability of S100A8/A9 alone, the improvement of S100A8/A9 combined with other analytes was marginal, except for a mild increase in Sn and Sp with MMP-9 (Table 2). Cognitive status remained unchanged for most of the patients with 6- and 12-month follow-up visits (57 and 28 remained in the non-CI and the CI groups, respectively, whereas 20 with CI changed to non-CI and 20 from non-CI to CI). S100A8/A9 serum levels increased over time in group 4 (from non- CI to CI) and there was a trend to reduced levels from the baseline and 6-month visits to the 12-month visit in group 3 (from CI to non-CI) (Figure 1).
Conclusion: In this large cohort of well-characterized SLE patients, amongst all measured analytes, S100A8/A9 had the greatest discriminatory ability in differentiating between SLE patients with and without CI. Replication of these findings is needed to confirm the utility of S100A8/A9 as a biomarker for CI in SLE. Research into the underlying mechanisms is also needed.
.jpg)
.jpg)
C. Munoz-Grajales: None; M. Barraclough: None; J. Diaz Martinez: None; J. Su: None; K. Bingham: None; M. Kakvan: None; R. Kretzmann: None; C. Tartaglia: None; L. Ruttan: None; M. Choi: AbbVie/Abbott, 2, 6, Amgen, 2, 6, AstraZeneca, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Celgene, 2, 6, Eli Lilly, 2, 6, GlaxoSmithKlein(GSK), 2, Janssen, 2, 6, Mallinckrodt, 2, Merck/MSD, 2, MitogenDx, 2, Organon, 6, Pfizer, 2, 6, Roche, 2, Werfen, 2; S. Appenzeller: None; S. Marzouk: None; D. Bonilla: None; P. Katz: None; D. Beaton: None; R. Green: None; J. Wither: AstraZeneca, 1, 6, Pfizer, 12, Indirect salary support through a Chair award to the Division of Rheumatology at the University of Toronto; Z. Touma: AstraZeneca, 2, GSK, 2.
Background/Purpose: Cognitive impairment (CI) is one of the most common manifestations of Neuropsychiatric Systemic Lupus Erythematosus (NPSLE). Studies have reported that SLE patients with different NPSLE syndromes have alterations in the cerebrospinal fluid or their serum levels of various cytokines and proteases (analytes) that can lead to neuroinflammation. However, studies focused on analytes in CI are scarce. In this study, we investigated the ability of various serum analytes to discriminate SLE patients with CI from those without impairment.
Methods: Two hundred ninety individuals 18-65 years old who met the 2019 EULAR/ACR classification criteria for SLE were included. Cognitive ability was measured by psychometrists utilizing the 1-hr ACR-Neuropsychological Battery (ACR-NB), and the scores were standardized by age and gender to operationally classify patients' performance into CI (z-score of ≤-1.5 in two or more domains) and non-CI. At the time of the neuropsychological assessment patients provided blood samples. The serum levels of nine analytes (IL-6, IL-10, IFN-γ, MMP-9, NGAL, S100A8/A9, S100B, TNF-α, and TWEAK) were determined using ELISA. The data were randomly partitioned into a training (70%) and a test (30%) sets. A predictive regression model was performed to evaluate the measured analytes' ability to discriminate SLE patients with CI from patients without impairment. The optimal cut-off values to discern between CI and non-CI for each analyte were obtained by Youden's index, and their sensitivity (Sn), specificity (Sp), and predictive values were calculated. For patients that had completed cognitive assessment at 6 and 12 months (n=125, 43%), pairwise comparisons of the S100A8/A9 serum levels between time points were performed.
Results: Of 290 patients, 40% had CI (n=116). Overall, no differences in demographic or clinical characteristics were observed between patients with and without CI. S100A8/A9 had the highest AUC (0.79, 95% CI: 0.66-0.90) (Table 1) and displayed the greatest discriminative ability (Sp 86% and PPV 82%) (Table 2) to differentiate between patients with and without CI. Compared to the predictive ability of S100A8/A9 alone, the improvement of S100A8/A9 combined with other analytes was marginal, except for a mild increase in Sn and Sp with MMP-9 (Table 2). Cognitive status remained unchanged for most of the patients with 6- and 12-month follow-up visits (57 and 28 remained in the non-CI and the CI groups, respectively, whereas 20 with CI changed to non-CI and 20 from non-CI to CI). S100A8/A9 serum levels increased over time in group 4 (from non- CI to CI) and there was a trend to reduced levels from the baseline and 6-month visits to the 12-month visit in group 3 (from CI to non-CI) (Figure 1).
Conclusion: In this large cohort of well-characterized SLE patients, amongst all measured analytes, S100A8/A9 had the greatest discriminatory ability in differentiating between SLE patients with and without CI. Replication of these findings is needed to confirm the utility of S100A8/A9 as a biomarker for CI in SLE. Research into the underlying mechanisms is also needed.
.jpg)
Table. 1. AUC of measured analytes serum levels to discriminate SLE patients with CI from those without CI.
.jpg)
Table 2. MMP-9, NGAL, S100A8/A9, and various combinations ability to discriminate SLE patients with CI from those without CI.

Figure 1. Pairwise comparison of S100A8/A9 serum levels between time points. P-values were adjusted using the Bonferroni multiple-testing correction method. Significant differences are indicated by asterisks (* p ≤ 0.05, **** p ≤ 0.0001). Non-significant differences are not displayed.
C. Munoz-Grajales: None; M. Barraclough: None; J. Diaz Martinez: None; J. Su: None; K. Bingham: None; M. Kakvan: None; R. Kretzmann: None; C. Tartaglia: None; L. Ruttan: None; M. Choi: AbbVie/Abbott, 2, 6, Amgen, 2, 6, AstraZeneca, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Celgene, 2, 6, Eli Lilly, 2, 6, GlaxoSmithKlein(GSK), 2, Janssen, 2, 6, Mallinckrodt, 2, Merck/MSD, 2, MitogenDx, 2, Organon, 6, Pfizer, 2, 6, Roche, 2, Werfen, 2; S. Appenzeller: None; S. Marzouk: None; D. Bonilla: None; P. Katz: None; D. Beaton: None; R. Green: None; J. Wither: AstraZeneca, 1, 6, Pfizer, 12, Indirect salary support through a Chair award to the Division of Rheumatology at the University of Toronto; Z. Touma: AstraZeneca, 2, GSK, 2.



