Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0582–0608) SLE – Treatment Poster I
0596: Deucravacitinib, an Oral, Selective, Allosteric Tyrosine Kinase 2 Inhibitor, in a Phase 2 Trial in Systemic Lupus Erythematosus (SLE): Achievement of Sustained SRI(4), BICLA and Dual Responses over 48 Weeks
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Richard Furie, MD
Chief of the Division of Rheumatology
Northwell Health
Manhasset, NY, NY, United StatesDisclosure(s): Biogen: Consultant (Ongoing), Grant/Research Support (Ongoing)
Abstract Poster Presenter(s)
Richard Furie1, Cristina Arriens2, Kenneth Kalunian3, Marilyn Pike4, Ronald van Vollenhoven5, Coburn Hobar6, Ayanbola Elegbe6, Samantha Pomponi6, Subhashis Banerjee6, Shalabh Singhal6, Thomas Wegman7 and Eric Morand8, 1Northwell Health, Manhasset, NY, 2Oklahoma Medical Research Foundation and University of Oklahoma Health Sciences Center, Department of Arthritis & Clinical Immunology, Oklahoma City, OK, 3University of California San Diego, La Jolla, CA, 4MedPharm consulting, Inc., Raleigh, NC, 5Amsterdam University Medical Centers, Amsterdam, Netherlands, 6Bristol Myers Squibb, Princeton, NJ, 7Bristol Myers Squibb, Beaver Falls, PA, 8Monash University, Centre for Inflammatory Diseases, Melbourne, Australia
Background/Purpose: Deucravacitinib is a first-in-class, oral, selective, allosteric tyrosine kinase 2 inhibitor approved in multiple countries for the treatment of adults with plaque psoriasis. In a phase 2 trial (NCT03252587) in patients with SLE receiving standard background therapy, deucravacitinib demonstrated efficacy vs placebo across multiple endpoints, including Systemic Lupus Erythematosus Responder Index-4 (SRI[4]) responses at week 32 (primary endpoint) and week 48 (secondary endpoint) as well as British Isles Lupus Assessment Group–based Composite Lupus Assessment (BICLA) responses at week 48 (secondary endpoint).1 Given that discordance between SRI(4) and BICLA responses has been reported with other agents,2 this post hoc analysis further evaluated efficacy, time to response, and sustained responses with deucravacitinib vs placebo for SRI(4) and BICLA in the phase 2 trial.
Methods: This 48-week, double-blind trial randomized 363 patients with active SLE 1:1:1:1 to placebo or deucravacitinib 3 mg twice daily (BID), 6 mg BID, or 12 mg once daily. Endpoints included the proportions of patients achieving SRI(4), BICLA, or simultaneous (dual) SRI(4)/BICLA responses at weeks 32 and 48, time to onset of responses, and proportions of patients with sustained responses through week 48 (responder at every visit from weeks 32 through 48). BICLA response, and therefore dual response, was measurable at the first visit after steroid taper completion (week 24 [day 168]). Analyses were descriptive.
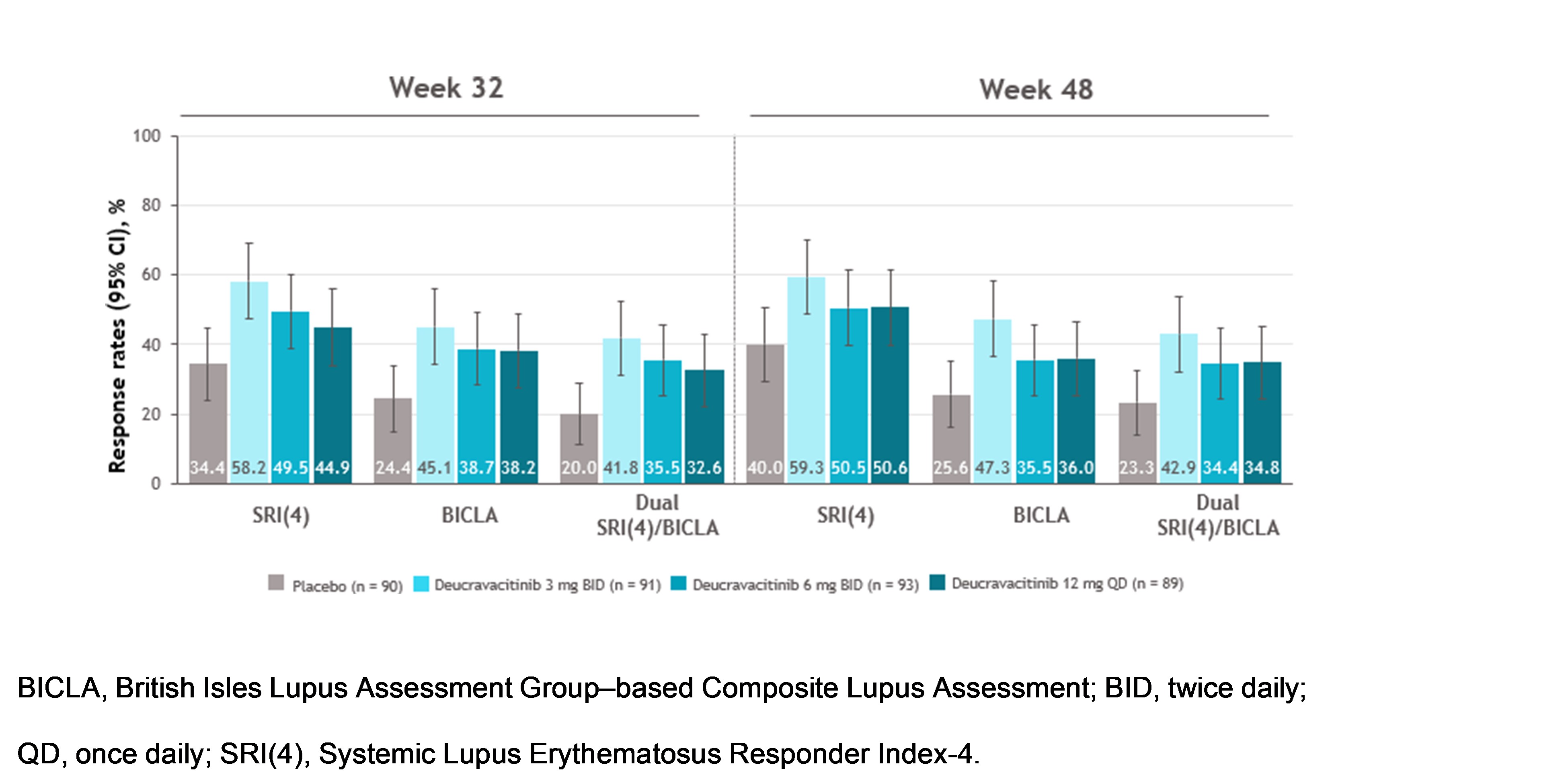
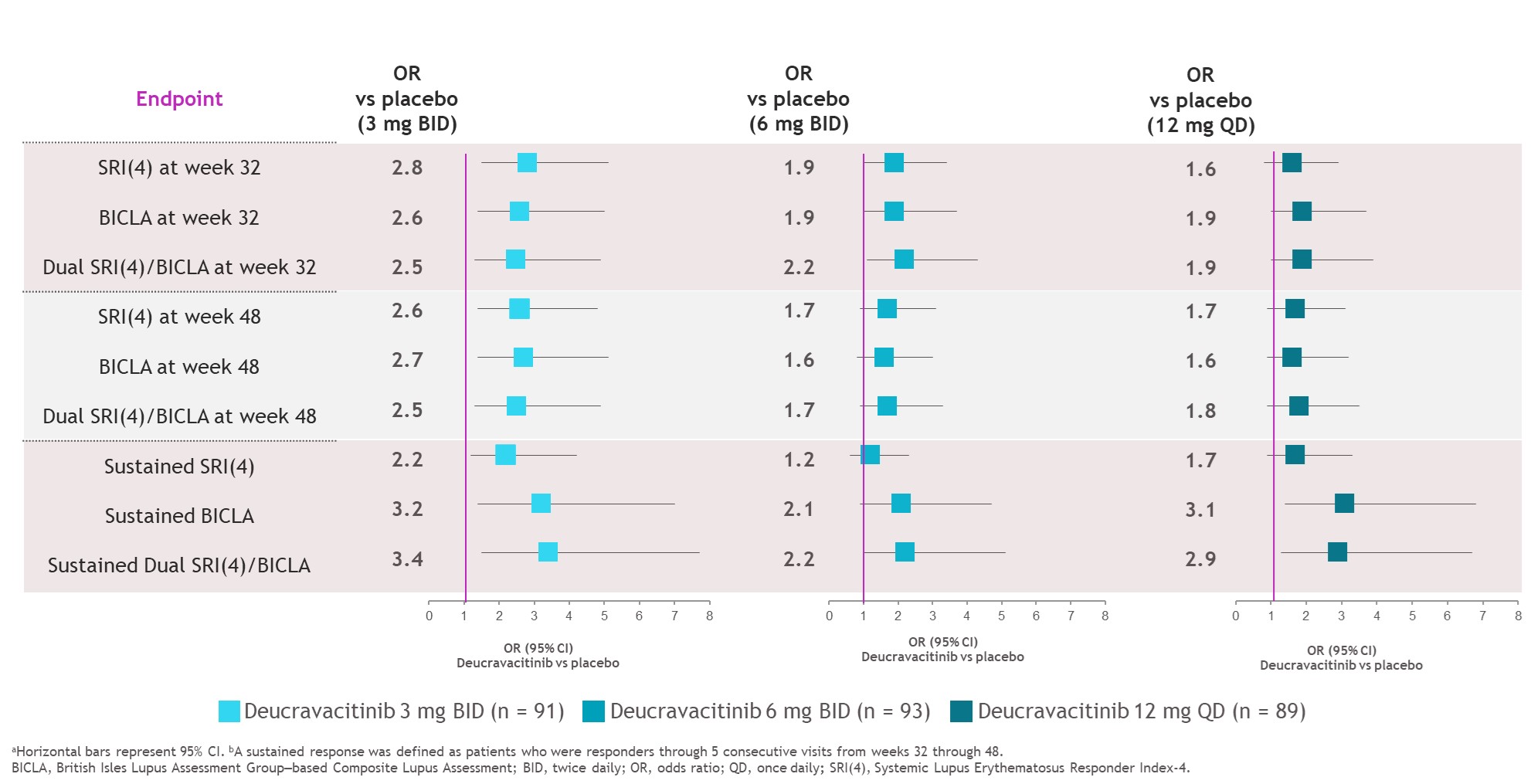
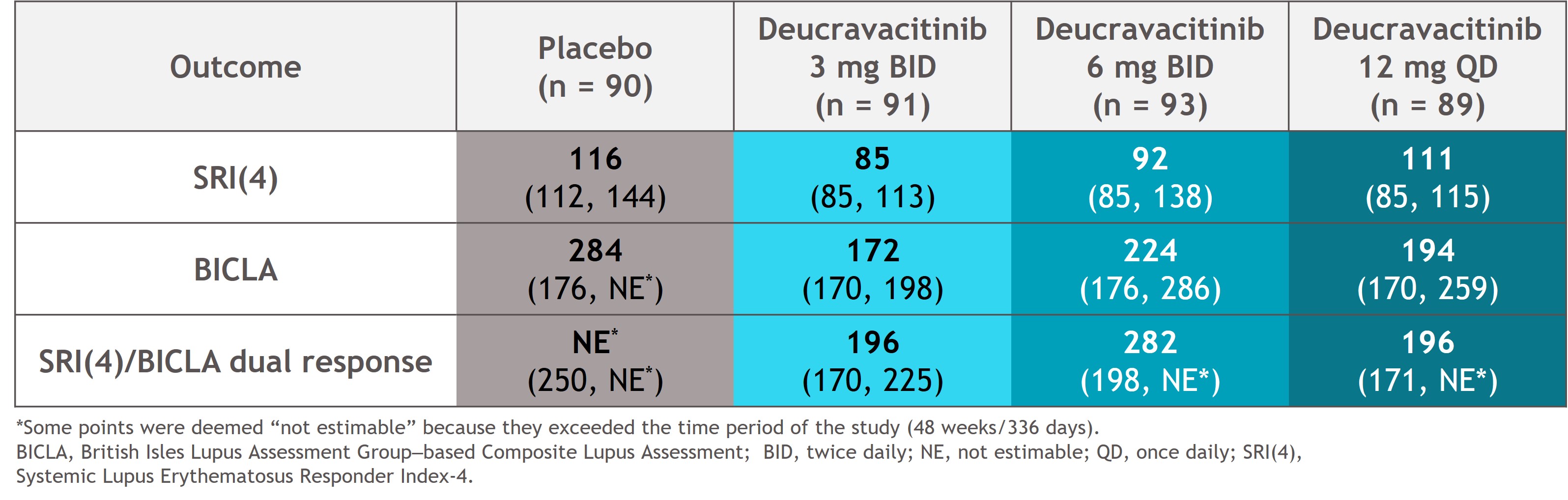
Results: At weeks 32 and 48, SRI(4), BICLA, and dual response rates were numerically higher with deucravacitinib vs placebo (Figure 1). Median time to onset of SRI(4), BICLA, and dual responses were shorter with deucravacitinib vs placebo, such that median times to onset of dual response were 196 to 282 days with deucravacitinib but was not reached for placebo (Table). Patients treated with deucravacitinib had a numerically higher likelihood of attaining SRI(4), BICLA, or dual SRI(4)/BICLA responses versus placebo at week 32, week 48, or sustained responses from week 32 through week 48 (Figure 2).
Conclusion: Deucravacitinib elicited higher response rates and faster time to SRI(4), BICLA, and dual responses vs placebo. Patients were more likely to sustain their treatment responses from weeks 32 through 48 with deucravacitinib vs placebo. Furthermore, there was consistency between SRI(4) and BICLA responses for patients treated with deucravacitinib. These data support the robust efficacy of deucravacitinib across multiple SLE response indices.
References
1. Morand E, et al. Arthritis Rheumatol. 2023;75(2):242-252.
2. Aguirre A, et al. Arthritis Rheumatol. 2022; 74 (suppl 9).
R. Furie: Biogen, 2, 5; C. Arriens: AstraZeneca, 1, 5, 6, Aurinia, 6, Bristol-Myers Squibb, 1, 5, Cabaletta, 1, GSK, 1, Kezar, 1, UCB, 1; K. Kalunian: AbbVie/Abbott, 2, Amgen, 5, AstraZeneca, 2, Aurinia, 2, Bristol-Myers Squibb(BMS), 2, Eli Lilly, 2, EquilliumBio, 2, Genentech, 2, Gilead, 2, Janssen, 2, KezarBio, 1, Merck/MSD, 2, Novartis, 2, Pfizer, 2, Remegene, 2, Roche, 2, UCB, 5; M. Pike: AstraZeneca, 2, Bristol-Myers Squibb(BMS), 2, Pfizer, 2, UCB, 2; R. van Vollenhoven: AbbVie, 2, 6, AstraZeneca, 2, 5, 6, Biogen, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Galapagos, 2, 5, 6, GlaxoSmithKline, 6, Janssen, 2, 6, MSD/Merck Sharp and Dohme, 5, Novartis, 5, Pfizer, 2, 5, 6, RemeGen, 2, Roche, 5, Sanofi, 5, UCB, 2, 5, 6; C. Hobar: Bristol-Myers Squibb(BMS), 3; A. Elegbe: Bristol-Myers Squibb(BMS), 3; S. Pomponi: Bristol-Myers Squibb(BMS), 3; S. Banerjee: Bristol-Myers Squibb(BMS), 3, 11; S. Singhal: Bristol-Myers Squibb(BMS), 3; T. Wegman: Bristol-Myers Squibb(BMS), 3, 12, Shareholder; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5.
Background/Purpose: Deucravacitinib is a first-in-class, oral, selective, allosteric tyrosine kinase 2 inhibitor approved in multiple countries for the treatment of adults with plaque psoriasis. In a phase 2 trial (NCT03252587) in patients with SLE receiving standard background therapy, deucravacitinib demonstrated efficacy vs placebo across multiple endpoints, including Systemic Lupus Erythematosus Responder Index-4 (SRI[4]) responses at week 32 (primary endpoint) and week 48 (secondary endpoint) as well as British Isles Lupus Assessment Group–based Composite Lupus Assessment (BICLA) responses at week 48 (secondary endpoint).1 Given that discordance between SRI(4) and BICLA responses has been reported with other agents,2 this post hoc analysis further evaluated efficacy, time to response, and sustained responses with deucravacitinib vs placebo for SRI(4) and BICLA in the phase 2 trial.
Methods: This 48-week, double-blind trial randomized 363 patients with active SLE 1:1:1:1 to placebo or deucravacitinib 3 mg twice daily (BID), 6 mg BID, or 12 mg once daily. Endpoints included the proportions of patients achieving SRI(4), BICLA, or simultaneous (dual) SRI(4)/BICLA responses at weeks 32 and 48, time to onset of responses, and proportions of patients with sustained responses through week 48 (responder at every visit from weeks 32 through 48). BICLA response, and therefore dual response, was measurable at the first visit after steroid taper completion (week 24 [day 168]). Analyses were descriptive.
Results: At weeks 32 and 48, SRI(4), BICLA, and dual response rates were numerically higher with deucravacitinib vs placebo (Figure 1). Median time to onset of SRI(4), BICLA, and dual responses were shorter with deucravacitinib vs placebo, such that median times to onset of dual response were 196 to 282 days with deucravacitinib but was not reached for placebo (Table). Patients treated with deucravacitinib had a numerically higher likelihood of attaining SRI(4), BICLA, or dual SRI(4)/BICLA responses versus placebo at week 32, week 48, or sustained responses from week 32 through week 48 (Figure 2).
Conclusion: Deucravacitinib elicited higher response rates and faster time to SRI(4), BICLA, and dual responses vs placebo. Patients were more likely to sustain their treatment responses from weeks 32 through 48 with deucravacitinib vs placebo. Furthermore, there was consistency between SRI(4) and BICLA responses for patients treated with deucravacitinib. These data support the robust efficacy of deucravacitinib across multiple SLE response indices.
References
1. Morand E, et al. Arthritis Rheumatol. 2023;75(2):242-252.
2. Aguirre A, et al. Arthritis Rheumatol. 2022; 74 (suppl 9).

Figure 1. SRI(4), BICLA, and dual SRI(4)/BICLA response rates at weeks 32 and 48

Figure 2. ORs versus placebo (a) for SRI(4), BICLA, and dual SRI(4)/BICLA at week 32, week 48, and sustained responses (b)

Table. Median time to first response for SRI(4), BICLA, and dual SRI(4)/BICLA
R. Furie: Biogen, 2, 5; C. Arriens: AstraZeneca, 1, 5, 6, Aurinia, 6, Bristol-Myers Squibb, 1, 5, Cabaletta, 1, GSK, 1, Kezar, 1, UCB, 1; K. Kalunian: AbbVie/Abbott, 2, Amgen, 5, AstraZeneca, 2, Aurinia, 2, Bristol-Myers Squibb(BMS), 2, Eli Lilly, 2, EquilliumBio, 2, Genentech, 2, Gilead, 2, Janssen, 2, KezarBio, 1, Merck/MSD, 2, Novartis, 2, Pfizer, 2, Remegene, 2, Roche, 2, UCB, 5; M. Pike: AstraZeneca, 2, Bristol-Myers Squibb(BMS), 2, Pfizer, 2, UCB, 2; R. van Vollenhoven: AbbVie, 2, 6, AstraZeneca, 2, 5, 6, Biogen, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Galapagos, 2, 5, 6, GlaxoSmithKline, 6, Janssen, 2, 6, MSD/Merck Sharp and Dohme, 5, Novartis, 5, Pfizer, 2, 5, 6, RemeGen, 2, Roche, 5, Sanofi, 5, UCB, 2, 5, 6; C. Hobar: Bristol-Myers Squibb(BMS), 3; A. Elegbe: Bristol-Myers Squibb(BMS), 3; S. Pomponi: Bristol-Myers Squibb(BMS), 3; S. Banerjee: Bristol-Myers Squibb(BMS), 3, 11; S. Singhal: Bristol-Myers Squibb(BMS), 3; T. Wegman: Bristol-Myers Squibb(BMS), 3, 12, Shareholder; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5.



