Poster Session A
Rheumatoid arthritis (RA)
Session: (0423–0459) RA – Treatment Poster I
0437: Comparative Safety of Biologic and Targeted Synthetic Disease Modifying Anti-Rheumatic Drugs for Cardiovascular Outcomes in Rheumatoid Arthritis
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- XS
Xavier Sendaydiego, MD
University of Washington
Seattle, WA, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Xavier Sendaydiego1, Laura Gold2, K Wysham3, Jean liew4, Maureen Dubreuil5, James Andrews1, Pankti Reid6, David Liew7, Radjiv Goulabchand8, Abha Singh9, Grant Hughes1, Mathilde Pioro1, Jeffrey Sparks10, Jeffrey Jarvik2, Siddharth Singh9 and Namrata Singh11, 1University of Washington, Seattle, WA, 2Department of Radiology and University of Washington Clinical Learning, Evidence, and Research (CLEAR) Center for Musculoskeletal Disorders, Seattle, WA, 3VA Puget Sound/University of Washington, Seattle, WA, 4Boston University, Boston, MA, 5Department of Rheumatology, Boston University School of Medicine, Milton, MA, 6University of Chicago Medical Center, Chicago, IL, 7Austin Health, Heidelberg, Australia, 8St. Eloi Hospital, Department of Internal Medicine and Multi-Organic Diseases, Montpellier, France, 9University of California San Diego, San Diego, CA, 10Division of Rheumatology, Inflammation, and Immunity, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, 11University of Washington, Bellevue, WA
Background/Purpose: Concern has arisen over the safety of Janus kinase inhibitors (JAKi) regarding cardiovascular (CV) outcomes in patients with rheumatoid arthritis (RA) with CV risk factors based on results of the ORAL surveillance trial (1). We compared the risk of major adverse cardiovascular events (MACE) between tumor necrosis factor inhibitors (TNFi), non-TNFi, and JAKi among patients with RA.
Methods: We performed a cohort study using Merative MarketScan databases (2012-2021) of RA patients (including those with pre-existing CV risk factors) identified using ≥1 ICD-9/10 codes, age 18-64 years, who initiated treatment with TNFi, non-TNFi, or JAKi. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for developing MACE (a composite of myocardial infarction, cardiac arrest, sudden death, stroke, percutaneous coronary intervention, coronary artery bypass graft, identified using ICD-9/10 codes and CPT codes) within 2 years of treatment initiation, adjusting for multiple confounders, including age, sex, region, comorbidity index, frailty (2), healthcare utilization, and surrogates for RA severity. Patients could contribute person-years to multiple drug exposures only if drug escalation mimicked typical clinical practice and were censored if they did not fill a prescription of these drugs for >90 days ( >180 days for rituximab due to its longer washout period) or their enrollment ended.
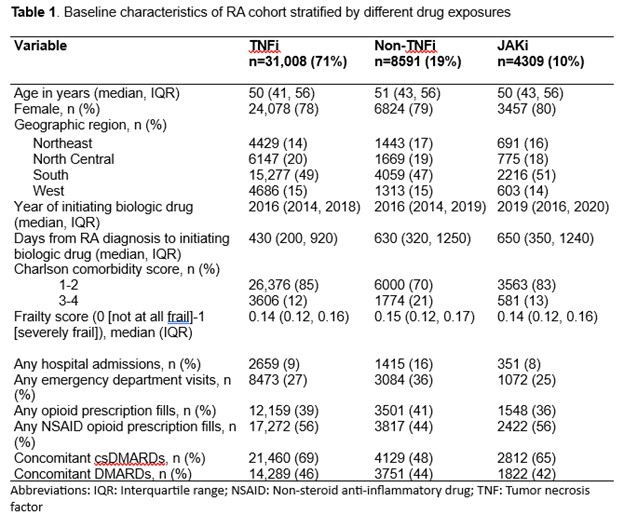
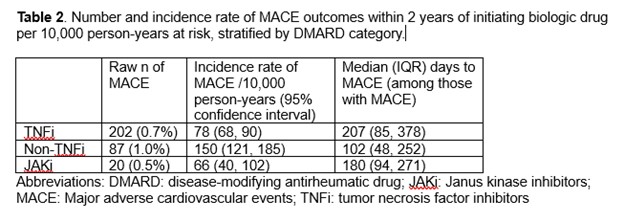
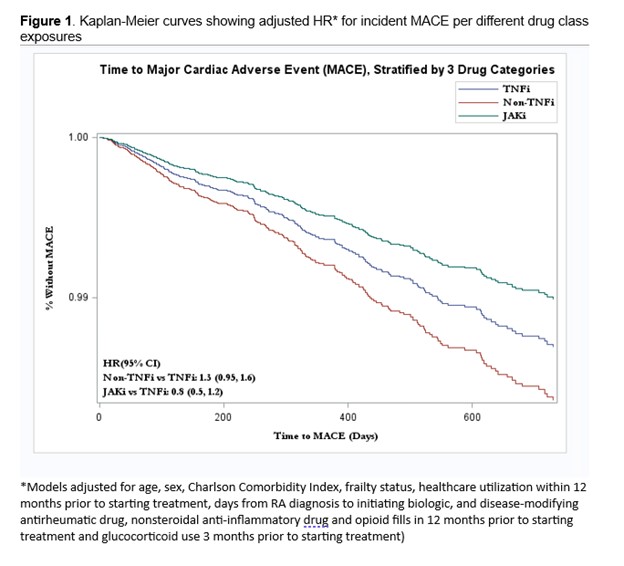
Results: A total of 40,207 patients met eligibility criteria for our study (71% initiated on TNFi, 19% non-TNFi, and 10% JAKi). Most patients were female (78-80%) with the mean age ranging from 47-49 years (Table 1). The median follow-up time was 230 days for TNFi, 190 days for non-TNFi, and 180 days for JAKi. The number of MACE per drug class was highest in non-TNFi exposures (Table 2). In multivariable models, patients taking non-TNFi had an HR (95% CI) 1.3 (0.95, 1.6) compared to those taking TNFi (Figure 1). Patients on JAKi had a similar risk of developing MACE (HR 0.8; 95% CI 0.5, 1.2) compared to those on TNFi, although this was statistically non-significant.
Conclusion: In this large nationwide study, although users of non-TNFi had increased risk of MACE, we did not detect any statistically significant difference overall. However, our study has limitations of residual confounding (e.g., inadequate accounting for RA severity using claims data, the fact that most RA patients initiate treatment with TNFi's before proceeding to non-TNFi's or JAKi's in clinical practice, and the small number of MACE outcomes in the study population). Additional studies with longer follow-up are needed for better assessment of MACE risk to guide clinical care.
References:
1. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386(4):316-26.
2. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980-7.
X. Sendaydiego: None; L. Gold: None; K. Wysham: None; J. liew: None; M. Dubreuil: Amgen, 2, Pfizer, 5, UCB Pharma, 2; J. Andrews: None; P. Reid: None; D. Liew: None; R. Goulabchand: Novartis, 2; A. Singh: AbbVie/Abbott, 5, Novartis, 5, Pfizer, 5; G. Hughes: Janssen, 3; M. Pioro: None; J. Sparks: AbbVie, 2, Amgen, 2, Boehringer Ingelheim, 2, Bristol-Myers Squibb, 2, 5, Gilead, 2, Inova Diagnostics, 2, Janssen, 2, Optum, 2, Pfizer, 2, ReCor, 2; J. Jarvik: GE Healthcare, 12, Travel reimbursement for Faculty Board of Review for GE-Association of University Radiologists Radiology Research Academic Fellowship (GERRAF), Springer Publishing, 9, Wolters Kluwer/UpToDate, 9; S. Singh: None; N. Singh: None.
Background/Purpose: Concern has arisen over the safety of Janus kinase inhibitors (JAKi) regarding cardiovascular (CV) outcomes in patients with rheumatoid arthritis (RA) with CV risk factors based on results of the ORAL surveillance trial (1). We compared the risk of major adverse cardiovascular events (MACE) between tumor necrosis factor inhibitors (TNFi), non-TNFi, and JAKi among patients with RA.
Methods: We performed a cohort study using Merative MarketScan databases (2012-2021) of RA patients (including those with pre-existing CV risk factors) identified using ≥1 ICD-9/10 codes, age 18-64 years, who initiated treatment with TNFi, non-TNFi, or JAKi. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for developing MACE (a composite of myocardial infarction, cardiac arrest, sudden death, stroke, percutaneous coronary intervention, coronary artery bypass graft, identified using ICD-9/10 codes and CPT codes) within 2 years of treatment initiation, adjusting for multiple confounders, including age, sex, region, comorbidity index, frailty (2), healthcare utilization, and surrogates for RA severity. Patients could contribute person-years to multiple drug exposures only if drug escalation mimicked typical clinical practice and were censored if they did not fill a prescription of these drugs for >90 days ( >180 days for rituximab due to its longer washout period) or their enrollment ended.
Results: A total of 40,207 patients met eligibility criteria for our study (71% initiated on TNFi, 19% non-TNFi, and 10% JAKi). Most patients were female (78-80%) with the mean age ranging from 47-49 years (Table 1). The median follow-up time was 230 days for TNFi, 190 days for non-TNFi, and 180 days for JAKi. The number of MACE per drug class was highest in non-TNFi exposures (Table 2). In multivariable models, patients taking non-TNFi had an HR (95% CI) 1.3 (0.95, 1.6) compared to those taking TNFi (Figure 1). Patients on JAKi had a similar risk of developing MACE (HR 0.8; 95% CI 0.5, 1.2) compared to those on TNFi, although this was statistically non-significant.
Conclusion: In this large nationwide study, although users of non-TNFi had increased risk of MACE, we did not detect any statistically significant difference overall. However, our study has limitations of residual confounding (e.g., inadequate accounting for RA severity using claims data, the fact that most RA patients initiate treatment with TNFi's before proceeding to non-TNFi's or JAKi's in clinical practice, and the small number of MACE outcomes in the study population). Additional studies with longer follow-up are needed for better assessment of MACE risk to guide clinical care.
References:
1. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386(4):316-26.
2. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980-7.



X. Sendaydiego: None; L. Gold: None; K. Wysham: None; J. liew: None; M. Dubreuil: Amgen, 2, Pfizer, 5, UCB Pharma, 2; J. Andrews: None; P. Reid: None; D. Liew: None; R. Goulabchand: Novartis, 2; A. Singh: AbbVie/Abbott, 5, Novartis, 5, Pfizer, 5; G. Hughes: Janssen, 3; M. Pioro: None; J. Sparks: AbbVie, 2, Amgen, 2, Boehringer Ingelheim, 2, Bristol-Myers Squibb, 2, 5, Gilead, 2, Inova Diagnostics, 2, Janssen, 2, Optum, 2, Pfizer, 2, ReCor, 2; J. Jarvik: GE Healthcare, 12, Travel reimbursement for Faculty Board of Review for GE-Association of University Radiologists Radiology Research Academic Fellowship (GERRAF), Springer Publishing, 9, Wolters Kluwer/UpToDate, 9; S. Singh: None; N. Singh: None.



