Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0582–0608) SLE – Treatment Poster I
0593: Lupus Nephritis of the Spanish National Registry of Belimumab in Patients with Systemic Lupus Erythematosus
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- VA
Vicente Aldasoro Caceres, MD
Hospital Universitario de Navarra
Pamplona, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
María laiño1, Monica Enguita2, Pablo Navarro3, Javier Loricera4, Angel Garcia-Aparicio5, Carmen Lasa6, Vanesa Calvo Río7, Adela Gallego8, Clara Moriano Morales9, Javier Narvaez10, MARIA IRENE CARRION BARBERA11, Jordi Camins-Fàbregas12, Joaquin Maria Belzunegui Otano13, Ana Urruticoechea14, Leticia del Olmo Perez15, Santos Castañeda16, Patricia Quiroga16, Ivette Casafont-Sole17, Juan Ramon De Dios Jimenez De Aber18, Marta López19, Judit Font Urgelles20, Rafaela Ortega Castro21, Marta Garijo Bufort22, Jorge Juan Fragio23, Ignacio Vázquez24, Mamen Ortega25, Aaron Fariñas26, Cilia Peralta27, Juan María Blanco28, Sergi Heredia Martin29, Maria Valvanera Pinillos30, Eztizen Labrador-Sánchez31, Piter Jose Cossio Jimenez32, Carlos Vazquez Galeano33 and Vicente Aldasoro1, 1Hospital Universitario de Navarra, Pamplona, Spain, 2Navarrabiomed-Unidad de Metodología, Pamplona, Spain, 3Rheumatology, Hospial Universitario Puerta de Hierro, Majadahonda, Spain, 4Hospital Universitario Marqués de Valdecilla, Santander, Spain, 5Hospital Universitario de Toledo, Toledo, Spain, 6Hospital Universitario Marqués de Valdecilla, IDIVAL., La Cavada, Spain, 7Valdecilla Hospital, Santander, Spain, 8Complejo Hospitalario Universitario de Badajoz, Badajoz, Spain, 9Rheumatology, Hospital Universitario de León, León, Spain, 10Hospital Universitario de Bellvitge, Barcelona, Spain, 11Hospital del Mar/Parc de Salut Mar-IMIM, Barcelona, Spain, 12Hospital General de Granollers, Granollers, Spain, 13University Hospital Donostia, Donostia-San Sebasti, Spain, 14Hospital Can Misses, Ibiza, Spain, 15Hospital Nuestra Señora del Prado, Talavera de la Reina, Spain, 16Hospital Universitario de la Princesa, Madrid, Spain, 17Hospital Universitari Germans Trias i Pujol, Badalona, Spain, 18Hospital de Araba, Alava, Spain, 19Hospital Universitario Arava, Pamplona, Spain, 20Hospital Universitario German Trias i Pujol,, Badalona, Spain, 21Hospital Reina Sofía, Cordoba, Spain, 22Hospital de Sagunto, Valencia, Spain, 23Hospital General Universitario Valencia, Valencia, Spain, 24H. U. Dr. Peset, Valencia, Spain, 25Hospital Universitario de Getafe, Getafe, Spain, 26Complejo Asistencial Universitario de Palencia, Palencia, Spain, 27Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain, 28Hospital Universitario de Basurto, Bilbao, Spain, 29Complex Hospitalari Moisès Broggi, Barcelona, Spain, 30Hospital Universitario San Pedro, Logroño, Spain, 31Hospital Universitario San Pedro, Laguardia, Spain, 32HOSPITAL REINA SOFIA, Tudela, Spain, 33ZARAGOZA, Madrid, Spain
Background/Purpose: Belimumab (BLM) is a B-cell stimulating factor (BlyS) monoclonal antibody, approved in 2022 for the treatment of lupus nephritis (NL) and since 2011 for systemic lupus erythematosus (SLE).
Objectives. To describe the demographic characteristics, efficacy and safety of BLM in real clinical practice in patients with lupus nephritis.
Methods: A descriptive, retrospective study of patients with lupus nephritis confirmed by biopsy from the Spanish multicenter registry of patients treated with BLM. Demographic, analytical and previous and concomitant treatment with belimumab were collected. To assess efficacy, analytical variables (anti-DNA, C3, C4), Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scale, and steroid doses at baseline and throughout follow-up were analyzed. Renal improvement was considered when a 24-hour proteinuria of less than 0.5 grams was reached. Safety profile of BLM was analyzed.
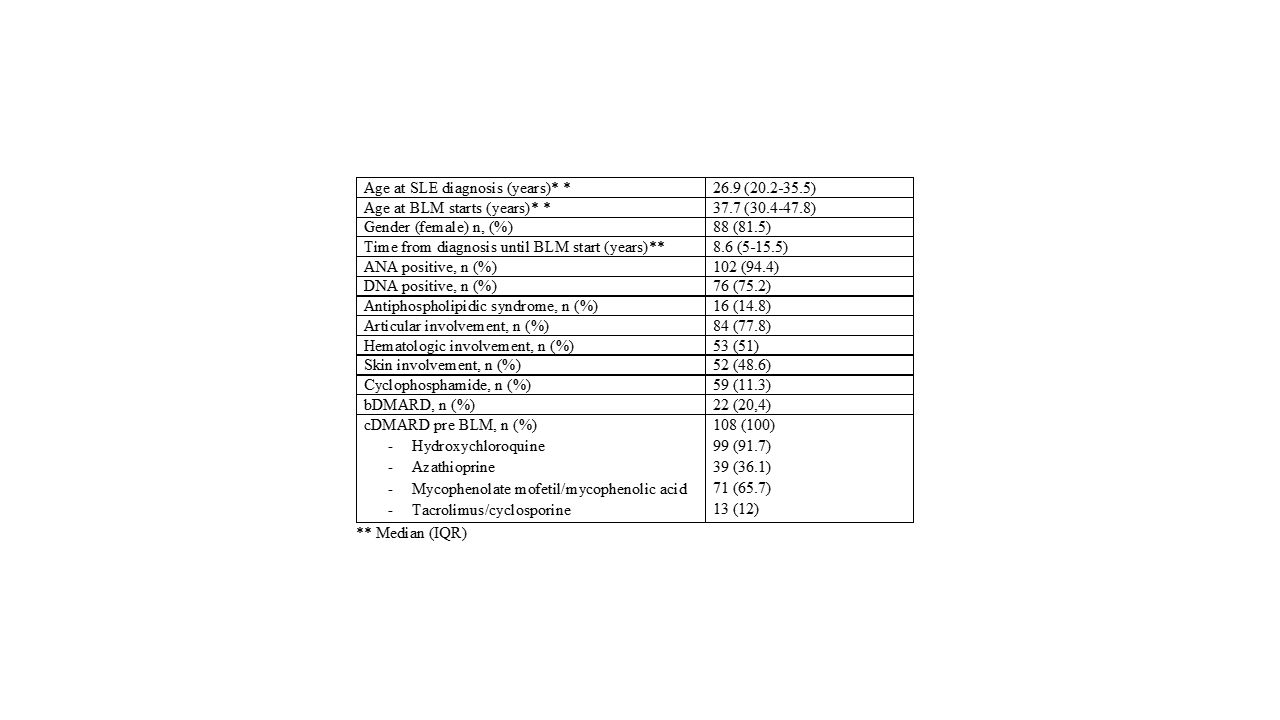
Results: We included 108 patients, 81.5% women, with a mean age at diagnosis of SLE of 26.9 years (20.2-35.5).The most frequent nephritis class was IV (47 patients, 43.5%), followed by class III (23 patients, 21.3%), V (19 patients, 17.6%) and I-II (19 patients, 17.6%). The time of evolution of the disease until the onset of BLM was 8.6 years (5-15.5).62 patients (57.4%) started BLM subcutaneously (SC), 33 patients (30.6%) intravenously (IV) and 13 patients (12%) change from IV to SC route. Baseline characteristics are summarized in Table 1
The median proteinuria in 24 hours was 1.0 (0.5 to 2.5). The median number of pre-BLM cDMARD uses was 2.0 (2.0-3.0), with antimalarials being the most commonly used (91.7%). 39 and 22 patients received cyclophosphamide and bDMARD prior to BLM respectively, being Rituximab (RTX) the most commonly bDMARD used. 107 patients received prednisone at the time of starting BLM with a median dose of 7.5 (5-1.5) mg.
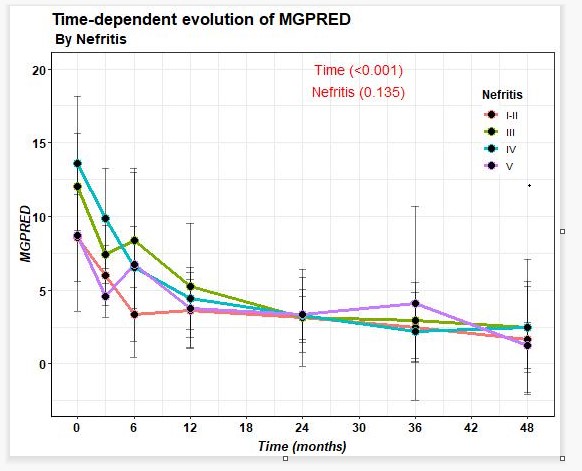
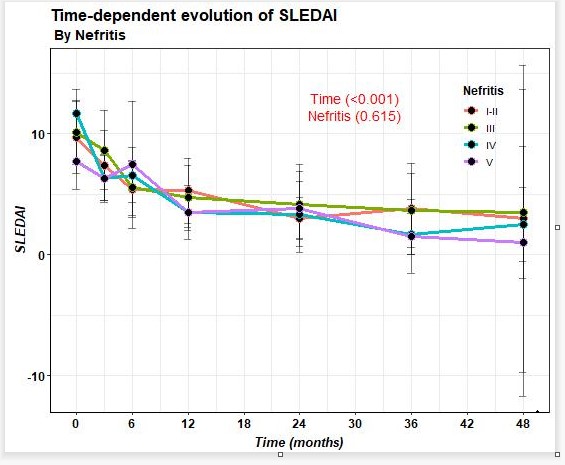
74.4% of patients improved after BLM in terms of reduction of proteinuria, with last 24-hour proteinuria of 0.2 (0.1-0.9) grams. A decrease in prednisone dose and SLEDAI improvement (Figure 1) was observed from baseline to last follow-up. On the other hand, a decrease in anti-DNA and an increase in C3 and C4 were observed.
21 patients (19.4%) discontinued treatment mainly due to ineffectiveness; 4 patients stopped BLM because of nephritis improvement. The median time on treatment for patients who had to discontinue BLM was 9 (6-24) months. In terms of safety, 28 patients had infections, most of them mild, being urine infection the most reported. One patient died due to meningitis. There were no tumors.
Conclusion: In this cohort of LN treated with BLM in real world settings, we observed an improvement of 24-hour proteinuria, SLEDAI, decrease of anti-DNA and increase of complement, despite of the administration route. No new safety alarms were reported.
M. laiño: None; M. Enguita: None; P. Navarro: None; J. Loricera: None; A. Garcia-Aparicio: None; C. Lasa: None; V. Calvo Río: None; A. Gallego: None; C. Moriano Morales: None; J. Narvaez: None; M. CARRION BARBERA: None; J. Camins-Fàbregas: None; J. Belzunegui Otano: None; A. Urruticoechea: None; L. del Olmo Perez: None; S. Castañeda: None; P. Quiroga: None; I. Casafont-Sole: None; J. Dios Jimenez De Aber: None; M. López: None; J. Font Urgelles: None; R. Ortega Castro: None; M. Garijo Bufort: None; J. Fragio: None; I. Vázquez: None; M. Ortega: None; A. Fariñas: None; C. Peralta: None; J. Blanco: None; S. Heredia Martin: None; M. Pinillos: None; E. Labrador-Sánchez: None; P. Cossio Jimenez: None; C. Vazquez Galeano: None; V. Aldasoro: None.
Background/Purpose: Belimumab (BLM) is a B-cell stimulating factor (BlyS) monoclonal antibody, approved in 2022 for the treatment of lupus nephritis (NL) and since 2011 for systemic lupus erythematosus (SLE).
Objectives. To describe the demographic characteristics, efficacy and safety of BLM in real clinical practice in patients with lupus nephritis.
Methods: A descriptive, retrospective study of patients with lupus nephritis confirmed by biopsy from the Spanish multicenter registry of patients treated with BLM. Demographic, analytical and previous and concomitant treatment with belimumab were collected. To assess efficacy, analytical variables (anti-DNA, C3, C4), Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scale, and steroid doses at baseline and throughout follow-up were analyzed. Renal improvement was considered when a 24-hour proteinuria of less than 0.5 grams was reached. Safety profile of BLM was analyzed.
Results: We included 108 patients, 81.5% women, with a mean age at diagnosis of SLE of 26.9 years (20.2-35.5).The most frequent nephritis class was IV (47 patients, 43.5%), followed by class III (23 patients, 21.3%), V (19 patients, 17.6%) and I-II (19 patients, 17.6%). The time of evolution of the disease until the onset of BLM was 8.6 years (5-15.5).62 patients (57.4%) started BLM subcutaneously (SC), 33 patients (30.6%) intravenously (IV) and 13 patients (12%) change from IV to SC route. Baseline characteristics are summarized in Table 1
The median proteinuria in 24 hours was 1.0 (0.5 to 2.5). The median number of pre-BLM cDMARD uses was 2.0 (2.0-3.0), with antimalarials being the most commonly used (91.7%). 39 and 22 patients received cyclophosphamide and bDMARD prior to BLM respectively, being Rituximab (RTX) the most commonly bDMARD used. 107 patients received prednisone at the time of starting BLM with a median dose of 7.5 (5-1.5) mg.
74.4% of patients improved after BLM in terms of reduction of proteinuria, with last 24-hour proteinuria of 0.2 (0.1-0.9) grams. A decrease in prednisone dose and SLEDAI improvement (Figure 1) was observed from baseline to last follow-up. On the other hand, a decrease in anti-DNA and an increase in C3 and C4 were observed.
21 patients (19.4%) discontinued treatment mainly due to ineffectiveness; 4 patients stopped BLM because of nephritis improvement. The median time on treatment for patients who had to discontinue BLM was 9 (6-24) months. In terms of safety, 28 patients had infections, most of them mild, being urine infection the most reported. One patient died due to meningitis. There were no tumors.
Conclusion: In this cohort of LN treated with BLM in real world settings, we observed an improvement of 24-hour proteinuria, SLEDAI, decrease of anti-DNA and increase of complement, despite of the administration route. No new safety alarms were reported.



M. laiño: None; M. Enguita: None; P. Navarro: None; J. Loricera: None; A. Garcia-Aparicio: None; C. Lasa: None; V. Calvo Río: None; A. Gallego: None; C. Moriano Morales: None; J. Narvaez: None; M. CARRION BARBERA: None; J. Camins-Fàbregas: None; J. Belzunegui Otano: None; A. Urruticoechea: None; L. del Olmo Perez: None; S. Castañeda: None; P. Quiroga: None; I. Casafont-Sole: None; J. Dios Jimenez De Aber: None; M. López: None; J. Font Urgelles: None; R. Ortega Castro: None; M. Garijo Bufort: None; J. Fragio: None; I. Vázquez: None; M. Ortega: None; A. Fariñas: None; C. Peralta: None; J. Blanco: None; S. Heredia Martin: None; M. Pinillos: None; E. Labrador-Sánchez: None; P. Cossio Jimenez: None; C. Vazquez Galeano: None; V. Aldasoro: None.



