Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes III: Disease Activity (2551–2556)
2556: End-of-Life in Systemic Lupus Erythematosus Beset by Increased Flares and Higher Treatment Burden: Data from a Prospective Large Multinational Cohort
Tuesday, November 14, 2023
5:15 PM - 5:25 PM PT
Location: Ballroom 20A
- JC
Jiacai Cho, MBBS, MRCP, MMed
National University Hospital
Singapore, SingaporeDisclosure information not submitted.
Presenting Author(s)
Jiacai Cho1, Liang Shen2, Rangi Kandane-Rathnayake3, Vera Golder3, Worawit Louthrenoo4, Yi-Hsing Chen5, Laniyati Hamijoyo6, Shue-Fen Luo7, Yeong-Jian J Wu8, Leonid Zamora9, Zhanguo Li10, Sargunan Sockalingam11, Yasuhiro Katsumata12, Masayoshi Harigai12, Yanjie Hao13, Zhuoli Zhang14, BMDB Basnayake15, Madelynn Chan16, Jun Kikuchi17, Tsutomu Takeuchi18, Sang-Cheol Bae19, Shereen Oon20, Sean O’Neill21, Fiona Goldblatt22, Kristine Ng23, Annie Law24, Nicola Tugnet25, Sunil Kumar26, Cherica Tee27, Michael Tee27, Naoaki Ohkubo28, Yoshiya Tanaka28, Sandra Navarra9, Chak Sing Lau29, Alberta Hoi30, Mandana Nikpour31, Eric Morand32 and Aisha Lateef33, 1National University Hospital, Singapore, Singapore, 2National University of Singapore, Singapore, Singapore, 3Monash University, Department of Medicine, Sub-faculty of Clinical and Molecular Medicine, Clayton, Australia, 4Chiang Mai University Hospital, Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai, Thailand, 5Taichung Veterans General Hospital, Taichung, Taiwan, 6Padjadjaran University/Hasan Sadikin General Hospital, Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Bandung, Indonesia, 7Chang Gung University, Department of Rheumatology, Allergy and Immunology, Chang Gung Memorial Hospital, Taoyuan, Taiwan, 8Chang Gung University, Department of Rheumatology, Allergy and Immunology, Chang Gung Memorial Hospital, Keelung, Taiwan, 9University of Santo Tomas Hospital, Joint and Bone Center, Manila, Philippines, 10Peking University People’s Hospital, Beijing, China, 11University of Malaya, Department of Medicine, Faculty of Medicine Building, Kuala Lumpur, Malaysia, 12Tokyo Women's Medical University, Division of Rheumatology, Department of Internal Medicine, Tokyo, Japan, 13The University of Melbourne Department of Medicine at St Vincents Hospital Melbourne, Melbourne, Australia, 14Peking University First Hospital, Rheumatology and Immunology Department, Beijing, China, 15Division of Nephrology, Teaching Hospital Kandy, Adelaide, Australia, 16Tan Tock Seng Hospital, Department of Rheumatology, Allergy & Immunology, Singapore, Singapore, 17Keio University, Keio, Japan, 18Keio University School of Medicine and Saitama Medical University, Tokyo, Japan, 19Hanyang University Hospital for Rheumatic Diseases and Hanyang University Institute for Rheumatology Research, Department of Rheumatology, Seoul, South Korea, 20Department of Medicine, The University of Melbourne at St Vincent’s Hospital, Melbourne, Australia, 21Department of Medicine, University of New South Wales, Kensington, Australia, 22Royal Adelaide Hospital and Flinders Medical Centre, Adelaide, Australia, 23Waitemata DHB, Auckland, New Zealand, 24Singapore General Hospital; Duke-NUS Medical School, Singapore, Singapore, 25Auckland District Health Board, Auckland, New Zealand, 26Middlemore Hospital, Auckland, New Zealand, 27University of the Philippines, Manila, Philippines, 28University of Occupational and Environmental Health, Kitakyushu, Japan, 29University of Hong Kong, Division of Rheumatology & Clinical Immunology, Department of Medicine, Queen Mary Hospital, Hong Kong, Hong Kong, 30Monash University, Department of Medicine, Sub-faculty of Clinical and Molecular Medicine, Melbourne, Australia, 31The University of Melbourne at St. Vincent’s Hospital Melbourne, Departments of Medicine and Rheumatology, Melbourne, Australia, 32Monash University, Centre for Inflammatory Diseases, Melbourne, Australia, 33National University Hospital, Rheumatology Division, Department of Medicine, Singapore, Singapore
Background/Purpose: SLE patients suffer high symptom burden at the end-of-life. However, the course of disease and treatment burden in the last year of life have not been described. Also, there remains few predictors to identify patients at risk of imminent death within a year that could trigger initiation of supportive care.
Methods: We collected data from SLE patients (ACR/SLICC criteria) prospectively between 2013 to 2020 from 13 Asia-pacific countries. Data at each visit included laboratory variables, SLEDAI and treatment. SLICC Damage Index (SDI) and 36-item Short Form Survey (SF-36) were administered annually. We captured causes of death, with each death possibly attributed to more than one cause. We computed a modified SLICC-frailty index that excluded Sjogren syndrome, hypothyroidism, BMI, hypertension and headache disorder. In order to build a model to identify patients in need of supportive care at the end-of-life, we used a generalized estimating equations (GEE) model that included variables at a given visit that could predict imminent death within a year. Next, we used GEE models to compare the course of disease in the last year of life versus before, with respect to (i) flares; (ii) use of immunosuppressive agents (IS); (iii) corticosteroid dose (CS); (iv) treatment escalation or tapering (any increase or decrease respecitvely, in IS/CS doses); (v) visit intervals; (vi) SF36 physical (PCS) and mental (MCS) scores.
Results: We studied 4105 patients, of which 90 died at a median age of 43 (29.5-56.5) years and 7 (2-12) years after diagnosis. The most prevalent cause of death was infection (57/90, 63.3%) followed by SLE (40/90, 44.4%). The modified SLICC frailty index scores before death revealed that 73/90 (81.1%) were least fit or frail. In the last year of life, patients spent 56.9% of days on IS, the median CS dose was 10 (2.2-17.8) mg/day and visit interval was 85 (50-117) days; 41/90 (53.9%) had treatment escalated whereas 16/90 (21.1%) received treatment tapering.
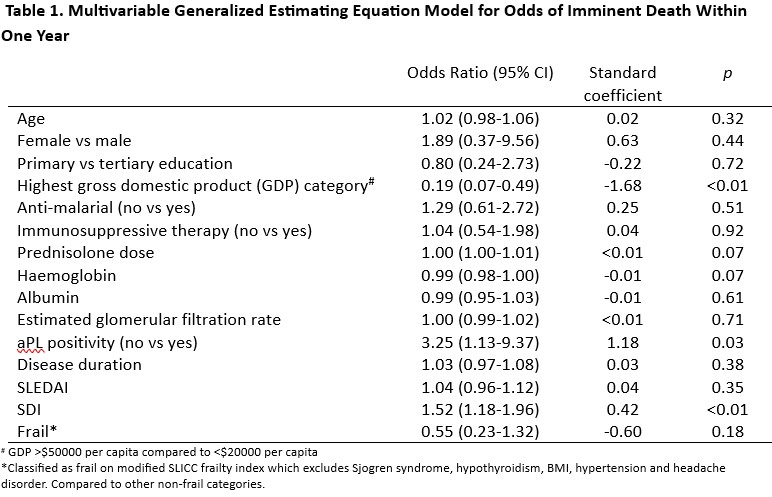
A model that included age, sex, anti-malarial use, country gross domestic product (GDP), haemoglobin, albumin, CS dose, SDI, SLEDAI and modified SLICC frailty was not sensitive (65.6%) or specific (73.4%) in identifying patients who would demise in a year. (Table 1).
In multivariable GEE models, the odds of flare were higher in the last year of life (OR 1.59, 95% CI 1.09-2.31, p=0.016). Patients had higher odds of staying in low disease activity (LLDAS) before the last year of life (OR 3.63, 95% CI 2.17-6.05, p< 0.01). Patients had fewer days on IS in the year preceding death, as compared to other years (p= 0.047), but had higher daily CS doses (p< 0.01) and shorter visit intervals (p< 0.01). Patients in the last year of life received more treatment escalations (OR 2.30, 95% CI 1.29-4.13, p=0.005). There were no significant differences in treatment tapering, PCS or MCS scores before or during the year preceding death.
Conclusion: In the year leading up to demise, SLE patients suffer increased flares and higher treatment burden. Existing disease-related instruments, laboratory and clinical variables have limited utility in identifying patients who would die within a year. Our results highlight an urgent need to better identify and support SLE patients near the end-of-life.
J. Cho: None; L. Shen: None; R. Kandane-Rathnayake: None; V. Golder: None; W. Louthrenoo: None; Y. Chen: None; L. Hamijoyo: None; S. Luo: None; Y. Wu: None; L. Zamora: None; Z. Li: None; S. Sockalingam: AstraZeneca, 2, 12, Other - for attending meetings and/or travel, Pfizer, 2, 12, Other - for attending meetings and/or travel, ZP Therapeutics, 2; Y. Katsumata: Asahi Kasei Pharma, 6, Astella, 6, AstraZeneca, 6, Chugai, 6, GlaxoSmithKlein(GSK), 6, Janssen, 6, Mitsubishi Tanabe Pharma Corporation, 6, Pfizer, 6; M. Harigai: Astellas Pharma, 6, AstraZeneca, 6, GlaxoSmithKlein(GSK), 6, 12, Post marketing surveillence, Novartis, 5; Y. Hao: None; Z. Zhang: None; B. Basnayake: None; M. Chan: None; J. Kikuchi: None; T. Takeuchi: AbbVie, 2, 5, 6, AYUMI, 5, Bristol-Myers Squibb, 6, Chugai, 2, 5, 6, Daiichi Sankyo, 5, Eisai, 5, 6, Eli Lilly Japan, 2, 6, Gilead, 2, 6, Janssen, 6, Mitsubishi-Tanabe, 2, 5, 6, ONO, 5, Pfizer Japan, 6, Taiho, 2; S. Bae: None; S. Oon: None; S. O’Neill: None; F. Goldblatt: None; K. Ng: AbbVie/Abbott, 1; A. Law: None; N. Tugnet: None; S. Kumar: None; C. Tee: None; M. Tee: None; N. Ohkubo: None; Y. Tanaka: AbbVie, 6, AstraZeneca, 6, BMS, 6, Boehringer-Ingelheim, 6, Chugai, 5, 6, Eisai, 5, 6, Eli Lilly, 6, Gilead, 6, GSK, 6, Mitsubishi-Tanabe, 5, Pfizer, 6, Taiho, 6, Taisho, 5, 6; S. Navarra: Astellas, 6, AstraZeneca, 6, Biogen, 2, Boehringer-Ingelheim, 2, GSK, 6, Novartis, 6, Pfizer, 6; C. Lau: AstraZeneca, 6, 12, external expert for SLE sterring committee 11.2022; A. Hoi: Abbvie, 6, AstraZeneca, 5, Australian Rheumatology Association, 4, Eli Lilly, 6, EUSA Pharma (UK) Limited, 2, Limbic, 6, Moose Republic, 6, Novartis, 6; M. Nikpour: AstraZeneca, 2, 6, Boehringer-Ingelheim, 2, 6, GSK, 2, 6, Janssen Pharmaceuticals, 2, 5, 6; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; A. Lateef: None.
Background/Purpose: SLE patients suffer high symptom burden at the end-of-life. However, the course of disease and treatment burden in the last year of life have not been described. Also, there remains few predictors to identify patients at risk of imminent death within a year that could trigger initiation of supportive care.
Methods: We collected data from SLE patients (ACR/SLICC criteria) prospectively between 2013 to 2020 from 13 Asia-pacific countries. Data at each visit included laboratory variables, SLEDAI and treatment. SLICC Damage Index (SDI) and 36-item Short Form Survey (SF-36) were administered annually. We captured causes of death, with each death possibly attributed to more than one cause. We computed a modified SLICC-frailty index that excluded Sjogren syndrome, hypothyroidism, BMI, hypertension and headache disorder. In order to build a model to identify patients in need of supportive care at the end-of-life, we used a generalized estimating equations (GEE) model that included variables at a given visit that could predict imminent death within a year. Next, we used GEE models to compare the course of disease in the last year of life versus before, with respect to (i) flares; (ii) use of immunosuppressive agents (IS); (iii) corticosteroid dose (CS); (iv) treatment escalation or tapering (any increase or decrease respecitvely, in IS/CS doses); (v) visit intervals; (vi) SF36 physical (PCS) and mental (MCS) scores.
Results: We studied 4105 patients, of which 90 died at a median age of 43 (29.5-56.5) years and 7 (2-12) years after diagnosis. The most prevalent cause of death was infection (57/90, 63.3%) followed by SLE (40/90, 44.4%). The modified SLICC frailty index scores before death revealed that 73/90 (81.1%) were least fit or frail. In the last year of life, patients spent 56.9% of days on IS, the median CS dose was 10 (2.2-17.8) mg/day and visit interval was 85 (50-117) days; 41/90 (53.9%) had treatment escalated whereas 16/90 (21.1%) received treatment tapering.
A model that included age, sex, anti-malarial use, country gross domestic product (GDP), haemoglobin, albumin, CS dose, SDI, SLEDAI and modified SLICC frailty was not sensitive (65.6%) or specific (73.4%) in identifying patients who would demise in a year. (Table 1).
In multivariable GEE models, the odds of flare were higher in the last year of life (OR 1.59, 95% CI 1.09-2.31, p=0.016). Patients had higher odds of staying in low disease activity (LLDAS) before the last year of life (OR 3.63, 95% CI 2.17-6.05, p< 0.01). Patients had fewer days on IS in the year preceding death, as compared to other years (p= 0.047), but had higher daily CS doses (p< 0.01) and shorter visit intervals (p< 0.01). Patients in the last year of life received more treatment escalations (OR 2.30, 95% CI 1.29-4.13, p=0.005). There were no significant differences in treatment tapering, PCS or MCS scores before or during the year preceding death.
Conclusion: In the year leading up to demise, SLE patients suffer increased flares and higher treatment burden. Existing disease-related instruments, laboratory and clinical variables have limited utility in identifying patients who would die within a year. Our results highlight an urgent need to better identify and support SLE patients near the end-of-life.

J. Cho: None; L. Shen: None; R. Kandane-Rathnayake: None; V. Golder: None; W. Louthrenoo: None; Y. Chen: None; L. Hamijoyo: None; S. Luo: None; Y. Wu: None; L. Zamora: None; Z. Li: None; S. Sockalingam: AstraZeneca, 2, 12, Other - for attending meetings and/or travel, Pfizer, 2, 12, Other - for attending meetings and/or travel, ZP Therapeutics, 2; Y. Katsumata: Asahi Kasei Pharma, 6, Astella, 6, AstraZeneca, 6, Chugai, 6, GlaxoSmithKlein(GSK), 6, Janssen, 6, Mitsubishi Tanabe Pharma Corporation, 6, Pfizer, 6; M. Harigai: Astellas Pharma, 6, AstraZeneca, 6, GlaxoSmithKlein(GSK), 6, 12, Post marketing surveillence, Novartis, 5; Y. Hao: None; Z. Zhang: None; B. Basnayake: None; M. Chan: None; J. Kikuchi: None; T. Takeuchi: AbbVie, 2, 5, 6, AYUMI, 5, Bristol-Myers Squibb, 6, Chugai, 2, 5, 6, Daiichi Sankyo, 5, Eisai, 5, 6, Eli Lilly Japan, 2, 6, Gilead, 2, 6, Janssen, 6, Mitsubishi-Tanabe, 2, 5, 6, ONO, 5, Pfizer Japan, 6, Taiho, 2; S. Bae: None; S. Oon: None; S. O’Neill: None; F. Goldblatt: None; K. Ng: AbbVie/Abbott, 1; A. Law: None; N. Tugnet: None; S. Kumar: None; C. Tee: None; M. Tee: None; N. Ohkubo: None; Y. Tanaka: AbbVie, 6, AstraZeneca, 6, BMS, 6, Boehringer-Ingelheim, 6, Chugai, 5, 6, Eisai, 5, 6, Eli Lilly, 6, Gilead, 6, GSK, 6, Mitsubishi-Tanabe, 5, Pfizer, 6, Taiho, 6, Taisho, 5, 6; S. Navarra: Astellas, 6, AstraZeneca, 6, Biogen, 2, Boehringer-Ingelheim, 2, GSK, 6, Novartis, 6, Pfizer, 6; C. Lau: AstraZeneca, 6, 12, external expert for SLE sterring committee 11.2022; A. Hoi: Abbvie, 6, AstraZeneca, 5, Australian Rheumatology Association, 4, Eli Lilly, 6, EUSA Pharma (UK) Limited, 2, Limbic, 6, Moose Republic, 6, Novartis, 6; M. Nikpour: AstraZeneca, 2, 6, Boehringer-Ingelheim, 2, 6, GSK, 2, 6, Janssen Pharmaceuticals, 2, 5, 6; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; A. Lateef: None.



