Poster Session A
Rheumatoid arthritis (RA)
Session: (0380–0422) RA – Diagnosis, Manifestations, and Outcomes Poster I
0390: Statins Influence the Relationship Between ATP-binding Cassette Transporter A1 (ABCA1)-mediated Cholesterol Efflux and Coronary Atherosclerosis in Rheumatoid Arthritis
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

George Karpouzas, MD (he/him/his)
Harbor-UCLA Medical Center
Torrance, CA, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
George Karpouzas1, Bianca Papotti2, Sarah Ormseth3, Marcella Palumbo2, Elizabeth Hernandez3, Maria Pia Adorni4, Francesca Zimetti2, Matthew Budoff1 and Nicoletta Ronda2, 1Harbor-UCLA Medical Center, Torrance, CA, 2University of Parma, Department of Food and Drug, Parma, Italy, 3The Lundquist Institute, Torrance, CA, 4Department of Medicine and Surgery, Unit of Neuroscience, University of Parma, Parma, Italy
Background/Purpose: Cholesterol efflux capacity (CEC) is the main antiatherogenic function of high-density lipoprotein (HDL). ATP-binding-cassette A1 (ABCA1) membrane transporter initiates cholesterol export from arterial macrophages to pre-β HDL particles fostering their maturation; in turn, those accept cholesterol through ABCG1-mediated export. Impaired pre-βHDL maturation may disrupt the collaborative function of the two transporters and adversely affect atherosclerosis. Statins exert atheroprotective functions systemically and locally on plaque. We here evaluated associations between ABCA1-CEC, coronary atherosclerosis and cardiovascular risk and the influence of statins on those relationships in rheumatoid arthritis (RA).
Methods: Atherosclerosis (noncalcified, partially or fully calcified, low attenuation plaques) was evaluated with coronary computed tomography angiography in 140 patients without cardiovascular disease and reassessed in 99 after 6.9±0.4 years. ABCA1-CEC and ABCG1-CEC were measured in J774 macrophages and Chinese hamster ovary cells respectively as previously described. Cox regression evaluated the association between ABCA1-CEC and cardiovascular risk. Multivariable negative binomial and robust logistic regression tested associations of ABCA1-CEC and its interactions with statin therapy on coronary plaque burden at baseline and its progression respectively.
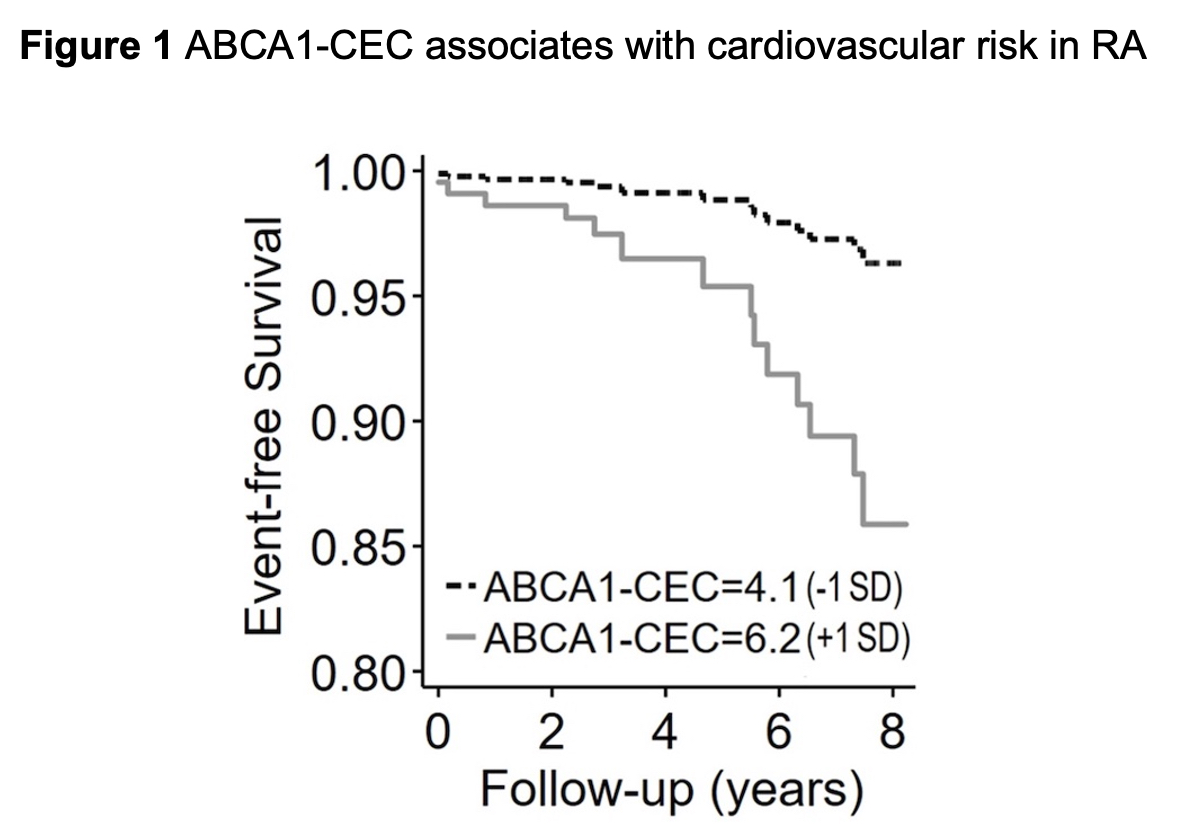
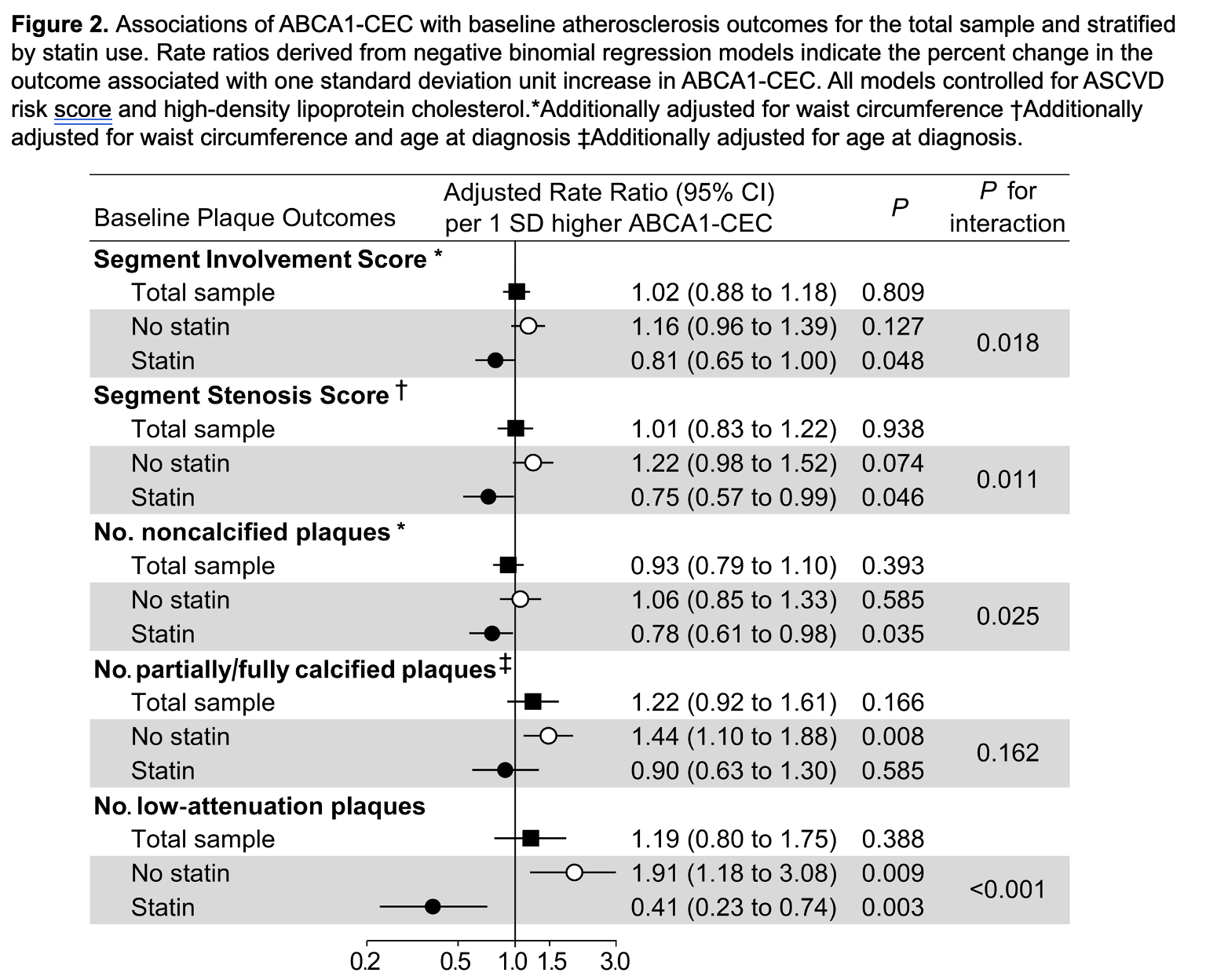
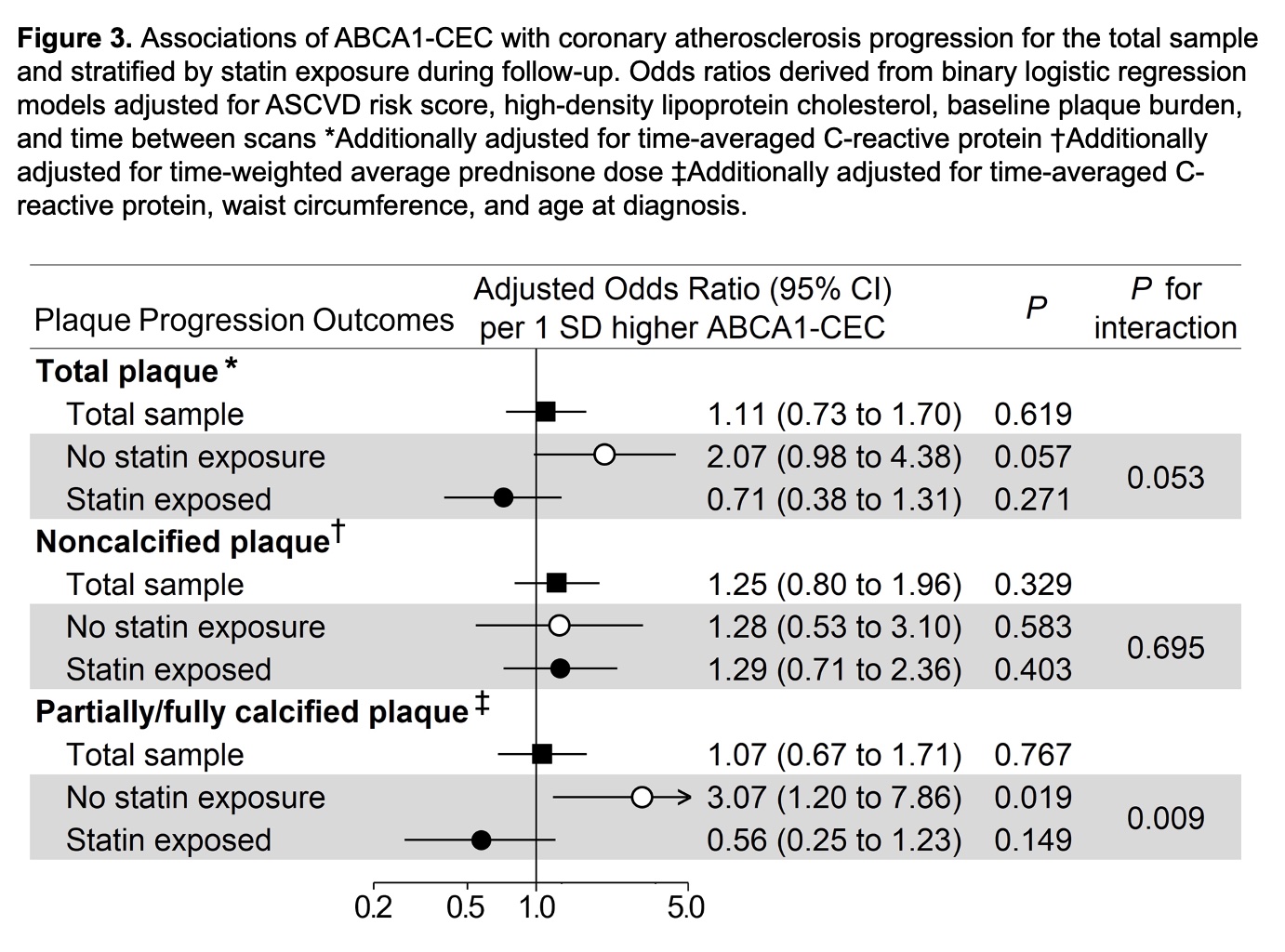
Results: ABCA1-CEC inversely correlated with ABCG1-CEC (Pearson r= -0.167, p=0.049). ABCA1-CEC (per SD increment) associated with long-term cardiovascular event risk after adjustments for cardiovascular risk score and baseline plaque burden [HR 2.05 (95% CI 1.20-3.48), Figure 1]. There was an interaction of ABCA1-CEC with time-varying statin use (p=0.038) such that current statin use inversely associated with risk only in patients with ABCA1-CEC below the upper tertile. ABCA1-CEC had no main effect on plaque or plaque progression; instead, ABCA1-CEC (per SD) associated with fewer baseline total plaques (adjusted rate ratio [aRR] 0.81, [95%CI 0.65-1.00]), noncalcified plaques (aRR 0.78 [95%CI 0.61-0.98]), and low attenuation plaques (aRR 0.41 [95%CI 0.23-0.74]) in statin users, and more low attenuation plaques (aRR 1.91 [95%CI 1.18-3.08]) in nonusers (p-for-interaction=0.018, 0.011, 0.025 and < 0.001 respectively, Figure 2). Moreover, ABCA1-CEC (per SD) associated with greater partially/fully-calcified plaque progression (adjusted odds ratio 3.07 [95%CI 1.20-7.86]) only in patients not exposed to statins during follow-up (p-for-interaction=0.009, Figure 3).
Conclusion: In the context of inflammation and impaired pre-β HDL maturation typical of RA, higher ABCA1-CEC may reflect a proatherogenic rather than atheroprotective state, associated with greater coronary atherosclerosis burden, vulnerability and cardiovascular risk. Statin use, by reducing cell cholesterol overload and restoring pre-β HDL maturation may unmask and promote the atheroprotective effect of ABCA1-CEC.
G. Karpouzas: Janssen, 1, Pfizer, 5, Scipher, 1; B. Papotti: None; S. Ormseth: None; M. Palumbo: None; E. Hernandez: None; M. Adorni: None; F. Zimetti: None; M. Budoff: None; N. Ronda: None.
Background/Purpose: Cholesterol efflux capacity (CEC) is the main antiatherogenic function of high-density lipoprotein (HDL). ATP-binding-cassette A1 (ABCA1) membrane transporter initiates cholesterol export from arterial macrophages to pre-β HDL particles fostering their maturation; in turn, those accept cholesterol through ABCG1-mediated export. Impaired pre-βHDL maturation may disrupt the collaborative function of the two transporters and adversely affect atherosclerosis. Statins exert atheroprotective functions systemically and locally on plaque. We here evaluated associations between ABCA1-CEC, coronary atherosclerosis and cardiovascular risk and the influence of statins on those relationships in rheumatoid arthritis (RA).
Methods: Atherosclerosis (noncalcified, partially or fully calcified, low attenuation plaques) was evaluated with coronary computed tomography angiography in 140 patients without cardiovascular disease and reassessed in 99 after 6.9±0.4 years. ABCA1-CEC and ABCG1-CEC were measured in J774 macrophages and Chinese hamster ovary cells respectively as previously described. Cox regression evaluated the association between ABCA1-CEC and cardiovascular risk. Multivariable negative binomial and robust logistic regression tested associations of ABCA1-CEC and its interactions with statin therapy on coronary plaque burden at baseline and its progression respectively.
Results: ABCA1-CEC inversely correlated with ABCG1-CEC (Pearson r= -0.167, p=0.049). ABCA1-CEC (per SD increment) associated with long-term cardiovascular event risk after adjustments for cardiovascular risk score and baseline plaque burden [HR 2.05 (95% CI 1.20-3.48), Figure 1]. There was an interaction of ABCA1-CEC with time-varying statin use (p=0.038) such that current statin use inversely associated with risk only in patients with ABCA1-CEC below the upper tertile. ABCA1-CEC had no main effect on plaque or plaque progression; instead, ABCA1-CEC (per SD) associated with fewer baseline total plaques (adjusted rate ratio [aRR] 0.81, [95%CI 0.65-1.00]), noncalcified plaques (aRR 0.78 [95%CI 0.61-0.98]), and low attenuation plaques (aRR 0.41 [95%CI 0.23-0.74]) in statin users, and more low attenuation plaques (aRR 1.91 [95%CI 1.18-3.08]) in nonusers (p-for-interaction=0.018, 0.011, 0.025 and < 0.001 respectively, Figure 2). Moreover, ABCA1-CEC (per SD) associated with greater partially/fully-calcified plaque progression (adjusted odds ratio 3.07 [95%CI 1.20-7.86]) only in patients not exposed to statins during follow-up (p-for-interaction=0.009, Figure 3).
Conclusion: In the context of inflammation and impaired pre-β HDL maturation typical of RA, higher ABCA1-CEC may reflect a proatherogenic rather than atheroprotective state, associated with greater coronary atherosclerosis burden, vulnerability and cardiovascular risk. Statin use, by reducing cell cholesterol overload and restoring pre-β HDL maturation may unmask and promote the atheroprotective effect of ABCA1-CEC.



G. Karpouzas: Janssen, 1, Pfizer, 5, Scipher, 1; B. Papotti: None; S. Ormseth: None; M. Palumbo: None; E. Hernandez: None; M. Adorni: None; F. Zimetti: None; M. Budoff: None; N. Ronda: None.



