Poster Session A
Rheumatoid arthritis (RA)
Session: (0380–0422) RA – Diagnosis, Manifestations, and Outcomes Poster I
0389: The Role of anti-CCP3 Antibodies in anti-CCP2 Antibody Negative Patients with Musculoskeletal Symptoms
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- AD
Andrea Di Matteo, MD, PhD (he/him/his)
Polytechnic University of Marche
Jesi, ItalyDisclosure information not submitted.
Abstract Poster Presenter(s)
Andrea Di Matteo1, Kulveer Mankia2, Leticia Garcia-Montoya2, Jacqueline Nam2, Sana sharrack3, Michael Mahler4 and Paul Emery3, 1Polytechnic University of Marche, Jesi, Italy, 2Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom, 3Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, and NIHR Leeds Biomedical Research Centre, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, 4Werfen, San Diego, CA
Background/Purpose: To investigate, in primary care, whether testing anti-CCP3 antibodies in anti-CCP2 negative individuals with musculoskeletal (MSK) symptoms, improved the prediction of inflammatory arthritis (IA)/rheumatoid arthritis (RA) progression.
Methods: A total of 469 anti-CCP2 negative individuals who presented to their general practitioner (GP) with new MSK symptoms were included in this study. All participants underwent baseline anti-CCP3 testing (QUANTA Lite CCP3; Inova Diagnostics) and received a questionnaire 12 months after enrolment assessing their disease status. The GPs of those individuals who reported progression to IA/RA were contacted by a rheumatologist to confirm the diagnosis. Univariate and multi-variate analyses were performed to establish variables associated with disease progression.
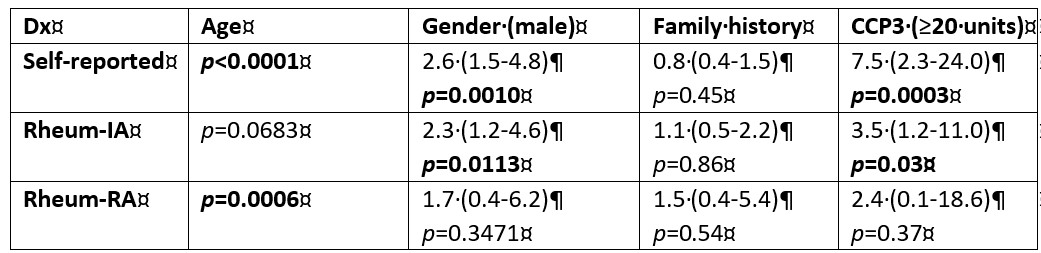
Results: Both the progression rate towards IA/RA and the prevalence of anti-CCP3 antibodies in anti-CCP2 negative individuals with MSK symptoms were low. Only 61/469 (13.0%) participants reported disease progression of which 43/61 (70.5%) and 13/61 (21.3%) were confirmed to have a diagnosis of IA and RA, respectively. Anti-CCP3 was positive in only 16/469 (3.4%) anti-CCP2 negative individuals. However, interestingly, in univariate analysis, anti-CCP3 positivity was associated with self-reported progression (p< 0.001) and with a diagnosis of IA (p=0.03), but not with a diagnosis of RA (p=0.37). In contrast, when considering antibody levels, anti-CCP3 differed significantly between progressors and non-progressors (p< 0.0001) for all three categories (self-reported progression, IA and RA diagnosis).
At the manufacturer's cut-off (≥20 units) the sensitivity for progression to IA/RA ranged from 8.0-14.0% with high specificity (≥97.0%). The corresponding odds ratios (OR) ranged from 2.4 (95% CI 0.3-20.0) to 7.8 (95% CI 2.8-21.8). Interestingly, when cut-offs were optimized for F-1 score, lower cut-off values (5 units) significantly increased the OR for progression in all three categories. After correcting for confounding factors (age, gender), in multi-variate analysis anti-CCP3 levels remained significantly associated with diagnosis of RA (p=0.02).
Conclusion: The rate of progression to IA/RA in anti-CCP2 negative individuals with MSK symptoms seen in primary care setting was low over a 12-months follow-up period. Our results showed that anti-CCP3 antibody levels have a potential role in improving prediction in IA/RA progression in anti-CCP2 negative individuals with MSK symptoms. Future studies are warranted to validate the cut-off values for anti-CCP3 antibodies with best prediction accuracy in this population.
A. Di Matteo: None; K. Mankia: Abbvie, 6, Eli Lilly, 5, Galapagos, 6, Gilead, 5, Serac Lifesciences, 6; L. Garcia-Montoya: None; J. Nam: None; S. sharrack: None; M. Mahler: werfen, 3; P. Emery: Boehringer Ingelheim, 2, Eli Lilly, 2, Novartis, 2.
Background/Purpose: To investigate, in primary care, whether testing anti-CCP3 antibodies in anti-CCP2 negative individuals with musculoskeletal (MSK) symptoms, improved the prediction of inflammatory arthritis (IA)/rheumatoid arthritis (RA) progression.
Methods: A total of 469 anti-CCP2 negative individuals who presented to their general practitioner (GP) with new MSK symptoms were included in this study. All participants underwent baseline anti-CCP3 testing (QUANTA Lite CCP3; Inova Diagnostics) and received a questionnaire 12 months after enrolment assessing their disease status. The GPs of those individuals who reported progression to IA/RA were contacted by a rheumatologist to confirm the diagnosis. Univariate and multi-variate analyses were performed to establish variables associated with disease progression.
Results: Both the progression rate towards IA/RA and the prevalence of anti-CCP3 antibodies in anti-CCP2 negative individuals with MSK symptoms were low. Only 61/469 (13.0%) participants reported disease progression of which 43/61 (70.5%) and 13/61 (21.3%) were confirmed to have a diagnosis of IA and RA, respectively. Anti-CCP3 was positive in only 16/469 (3.4%) anti-CCP2 negative individuals. However, interestingly, in univariate analysis, anti-CCP3 positivity was associated with self-reported progression (p< 0.001) and with a diagnosis of IA (p=0.03), but not with a diagnosis of RA (p=0.37). In contrast, when considering antibody levels, anti-CCP3 differed significantly between progressors and non-progressors (p< 0.0001) for all three categories (self-reported progression, IA and RA diagnosis).
At the manufacturer's cut-off (≥20 units) the sensitivity for progression to IA/RA ranged from 8.0-14.0% with high specificity (≥97.0%). The corresponding odds ratios (OR) ranged from 2.4 (95% CI 0.3-20.0) to 7.8 (95% CI 2.8-21.8). Interestingly, when cut-offs were optimized for F-1 score, lower cut-off values (5 units) significantly increased the OR for progression in all three categories. After correcting for confounding factors (age, gender), in multi-variate analysis anti-CCP3 levels remained significantly associated with diagnosis of RA (p=0.02).
Conclusion: The rate of progression to IA/RA in anti-CCP2 negative individuals with MSK symptoms seen in primary care setting was low over a 12-months follow-up period. Our results showed that anti-CCP3 antibody levels have a potential role in improving prediction in IA/RA progression in anti-CCP2 negative individuals with MSK symptoms. Future studies are warranted to validate the cut-off values for anti-CCP3 antibodies with best prediction accuracy in this population.

Demographic and serological features and their association with disease progression.
A. Di Matteo: None; K. Mankia: Abbvie, 6, Eli Lilly, 5, Galapagos, 6, Gilead, 5, Serac Lifesciences, 6; L. Garcia-Montoya: None; J. Nam: None; S. sharrack: None; M. Mahler: werfen, 3; P. Emery: Boehringer Ingelheim, 2, Eli Lilly, 2, Novartis, 2.



