Poster Session A
Osteoarthritis (OA) and related disorders
Session: (0308–0324) Osteoarthritis – Clinical Poster I
0317: Long-term Effectiveness of a Lifestyle Program for Osteoarthritis: One-year Follow-up of the “Plants for Joints” Randomized Clinical Trial
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MG
Abstract Poster Presenter(s)
Carlijn Wagenaar1, Wendy Walrabenstein1, Marike Van der Leeden2, Martijn Gerritsen2, Jos W.R. Twisk1, Martin van der Esch2, Henriët van Middendorp3, Peter Weijs4 and Dirkjan van Schaardenburg5, 1Amsterdam University Medical Centers, Amsterdam, Netherlands, 2Reade Rheumatology Center, Amsterdam, Netherlands, 3Leiden University, Leiden, Netherlands, 4Amsterdam University of Applied Sciences, Amsterdam, Netherlands, 5Reade, Amsterdam, Netherlands
Background/Purpose: The 16-week Plants for Joints (PFJ) multidisciplinary lifestyle program, based on a whole-food plant-based diet, physical activity, and stress management, significantly reduced The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score compared to usual care in people with hip and/or knee osteoarthritis (OA) and metabolic syndrome.1,2 The objective was to determine the long-term effectiveness of the PFJ program on pain, stiffness, and function in people with OA.
Methods: People with knee and/or hip OA and metabolic syndrome were randomized to receive the PFJ program in addition to usual care, or the control group which received usual care. After this 16-week RCT period the control group also received the program. After completion of the program all participants were followed-up for one year with biannual visits and 6 adherence-promoting webinars. Medication changes (pain, cholesterol, and diabetes medication) were assessed at one year as an "increase," "stable," or "decrease" compared to baseline. Secondary outcomes included anthropometric and metabolic markers. An intention-to-treat analysis with a linear mixed model was used to analyze within-group differences.
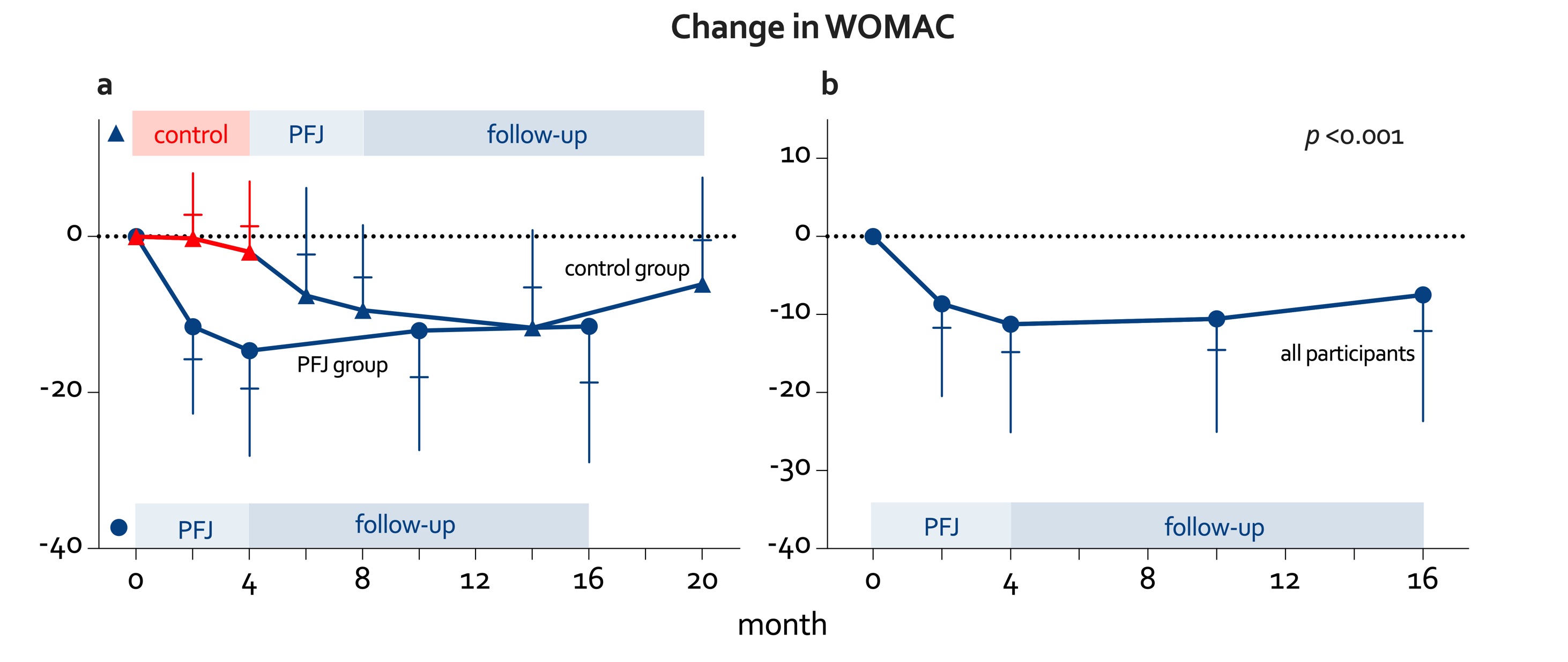
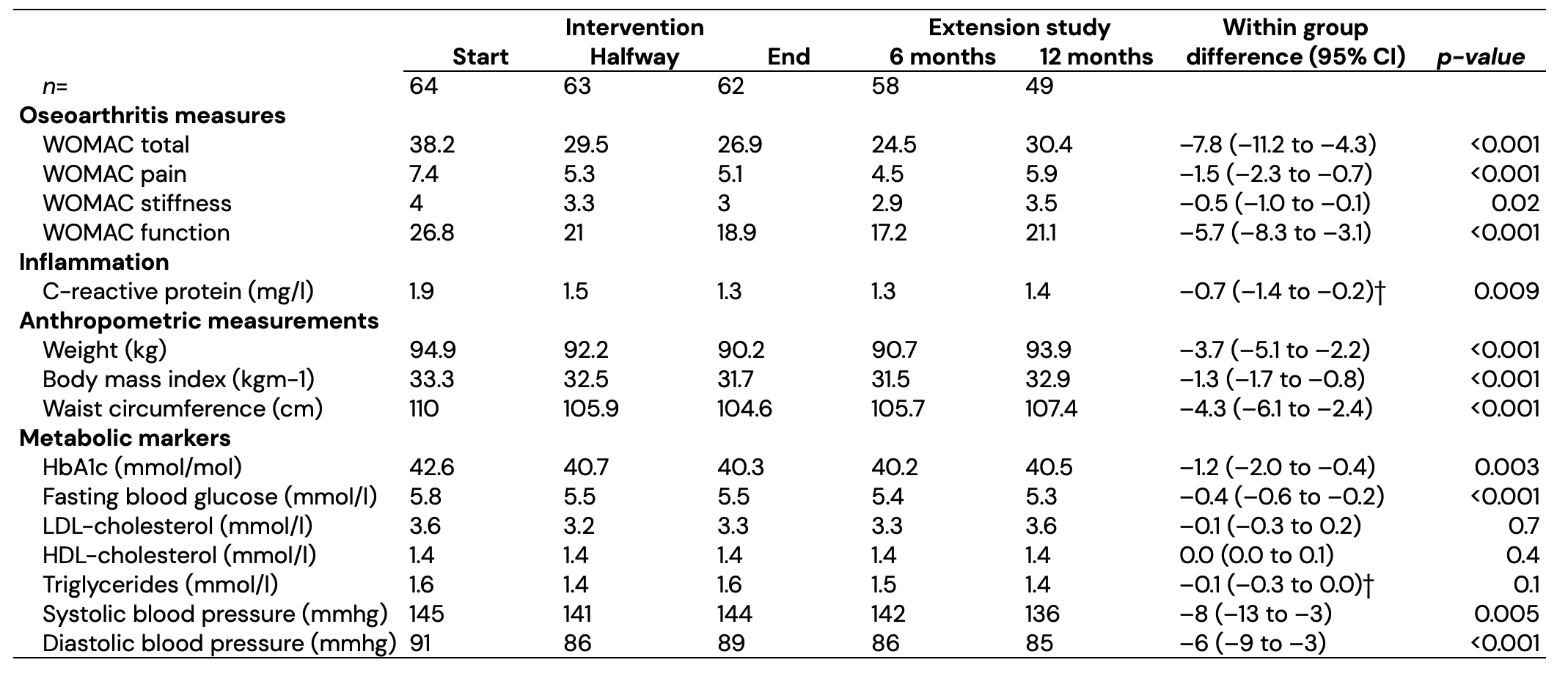
Results: 49 of 64 participants (77%), who completed the initial 16-week clinical trial, completed the one-year follow-up. 84% of participants were female with a mean (SD) age of 63 (6) and body mass index of 33 (5) kg/m². In the year after completing the PFJ program the WOMAC score was lower, yet increased slightly again, whereby a mean –7.8-point difference was observed after one year compared to baseline values (p < 0.001) (Figure 1). All components of the WOMAC improved significantly compared to before the program (Table 1). Of the 18 participants who completed the follow-up and used pain medication, 11 (61%) decreased or stopped, 3 had stable, and 4 had increased medication. After the 1-year follow-up period weight, waist circumference, HbA1c, CRP, and blood pressure became or remained significantly lower than baseline values, although there was no longer a significant difference in LDL cholesterol. Furthermore, of those completing the follow-up, 3 (33%) and 8 (44%) participants decreased diabetes and cholesterol medication respectively while only 2 participants had increased medication.
Conclusion: The PFJ lifestyle program significantly decreased pain, stiffness, and improved function in people with OA and metabolic syndrome and its effects were largely sustained till one year after program completion with reduced pain medication. Metabolic benefits found after the lifestyle intervention were partially sustained, possibly indicating attenuated adherence to the program in the follow-up.
References
1. Walrabenstein, Trials 2021
2. Walrabenstein, Osteoarthritis and Cartilage 2023
C. Wagenaar: The Netherlands Organisation for Health Research and Development (ZonMw), 5; W. Walrabenstein: None; M. Van der Leeden: None; M. Gerritsen: Horizon Therapeutics, 5; J. Twisk: None; M. van der Esch: None; H. van Middendorp: None; P. Weijs: None; D. van Schaardenburg: None.
Background/Purpose: The 16-week Plants for Joints (PFJ) multidisciplinary lifestyle program, based on a whole-food plant-based diet, physical activity, and stress management, significantly reduced The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score compared to usual care in people with hip and/or knee osteoarthritis (OA) and metabolic syndrome.1,2 The objective was to determine the long-term effectiveness of the PFJ program on pain, stiffness, and function in people with OA.
Methods: People with knee and/or hip OA and metabolic syndrome were randomized to receive the PFJ program in addition to usual care, or the control group which received usual care. After this 16-week RCT period the control group also received the program. After completion of the program all participants were followed-up for one year with biannual visits and 6 adherence-promoting webinars. Medication changes (pain, cholesterol, and diabetes medication) were assessed at one year as an "increase," "stable," or "decrease" compared to baseline. Secondary outcomes included anthropometric and metabolic markers. An intention-to-treat analysis with a linear mixed model was used to analyze within-group differences.
Results: 49 of 64 participants (77%), who completed the initial 16-week clinical trial, completed the one-year follow-up. 84% of participants were female with a mean (SD) age of 63 (6) and body mass index of 33 (5) kg/m². In the year after completing the PFJ program the WOMAC score was lower, yet increased slightly again, whereby a mean –7.8-point difference was observed after one year compared to baseline values (p < 0.001) (Figure 1). All components of the WOMAC improved significantly compared to before the program (Table 1). Of the 18 participants who completed the follow-up and used pain medication, 11 (61%) decreased or stopped, 3 had stable, and 4 had increased medication. After the 1-year follow-up period weight, waist circumference, HbA1c, CRP, and blood pressure became or remained significantly lower than baseline values, although there was no longer a significant difference in LDL cholesterol. Furthermore, of those completing the follow-up, 3 (33%) and 8 (44%) participants decreased diabetes and cholesterol medication respectively while only 2 participants had increased medication.
Conclusion: The PFJ lifestyle program significantly decreased pain, stiffness, and improved function in people with OA and metabolic syndrome and its effects were largely sustained till one year after program completion with reduced pain medication. Metabolic benefits found after the lifestyle intervention were partially sustained, possibly indicating attenuated adherence to the program in the follow-up.
References
1. Walrabenstein, Trials 2021
2. Walrabenstein, Osteoarthritis and Cartilage 2023

Figure 1. Mean change in WOMAC (a) during the randomized controlled trial phase and one-year follow-up period per trial arm and (b) for the whole cohort before and after completing the lifestyle intervention and one year follow-up.

Table 1. Plants for Joints cohort at start and end of the 16-week intervention period as well as during the one year extension study (6 and 12 months after completing the intervention). Continuous variables reported as mean (SD) when normally distributed or as median [IQR] when skewed. Within-group difference shown between start of the lifestyle intervention and end of the 12-month follow-up determined using the linear-mixed model when model assumptions were met. For variables in which model assumptions were not met (†) a linear-mixed model was performed after log transformation and within group differences were reported as median difference of complete paired values determined using a Wilcoxon test (p-values from the linear mixed model are shown, all were similar to the Wilcoxon test).
C. Wagenaar: The Netherlands Organisation for Health Research and Development (ZonMw), 5; W. Walrabenstein: None; M. Van der Leeden: None; M. Gerritsen: Horizon Therapeutics, 5; J. Twisk: None; M. van der Esch: None; H. van Middendorp: None; P. Weijs: None; D. van Schaardenburg: None.



