Poster Session A
Rheumatoid arthritis (RA)
Session: (0423–0459) RA – Treatment Poster I
0432: Predictors of Use, Survival and Safety of Tofacitinib Monotherapy vs. in Combination with CsDMARDs in Daily Clinical Practice
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- RP
Rodolfo Perez-Alamino, MD (he/him/his)
Hospital Avellaneda
Tucuman, ArgentinaDisclosure information not submitted.
Abstract Poster Presenter(s)
Rodolfo Perez-Alamino1, Carolina Isnardi2, Enrique Soriano3, Luciano Lo Giudice4, johana zacariaz hereter4, Gustavo Casado5, Victor Caputo5, Andrea Schmichowski5, Cecilia Romeo5, Estela Rivero5, Florencia Savy6, Mercedes García7, Olga Romano1, Hernan Maldonado Ficco8 and Gustavo Citera2, 1Hospital Avellaneda, Tucuman, Argentina, 2IREP, Buenos Aires, Argentina, 3Rheumatology Section, Internal Medicine Services, Hospital Italiano de Buenos Aires, and University Institute Hospital Italiano de Buenos Aires, Buenos Aires, Argentina, 4Hospital Italiano de Buenos Aires, Buenos Aires, Argentina, 5Hospital Militar Central, Buenos Aires, Argentina, 6HIGA San Martin, La Plata, Argentina, 7Hospital Interzonal General de Agudos José de San Martín, La Plata, Argentina, 8Hospital San Antonio de Padua, Río Cuarto, Argentina
Background/Purpose: Different guidelines recommend the use of tofacitinib either in monotherapy or combined with methotrexate (combination therapy) in patients with rheumatoid arthritis (RA) with an inadequate response to conventional synthetic DMARDs (csDMARDs) or biologic DMARDs (bDMARDs). Although the evidence originating from clinical trials does not show notable differences in terms of efficacy and safety with both strategies, data from real life is limited.
To evaluate the frequency, predictors of use and survival of tofacitinib in monotherapy compared with combination therapy in a cohort of patients with RA from daily clinical practice in our country.
Methods: Longitudinal, observational study which includes a cohort of patients with RA (ACR/EULAR 2010 criteria) from different rheumatology centers who started treatment with tofacitinib. Patients were evaluated at baseline and followed up every six months from the start of medication. Sociodemographic, clinical and treatment data were collected. Chi2 test, Fisher's exact test, Student's T test and Mann Whitney were used according to the nature of the variables. Kaplan-Meier method and the Log Rank test were applied to compare survival of treatment in monotherapy vs. combination therapy. Cox regression analysis was used to evaluate variables associated with lower survival. The incidence of adverse events (AEs) was calculated as events every 100 patients/year (p/y). A p< 0.05 was considered significant.
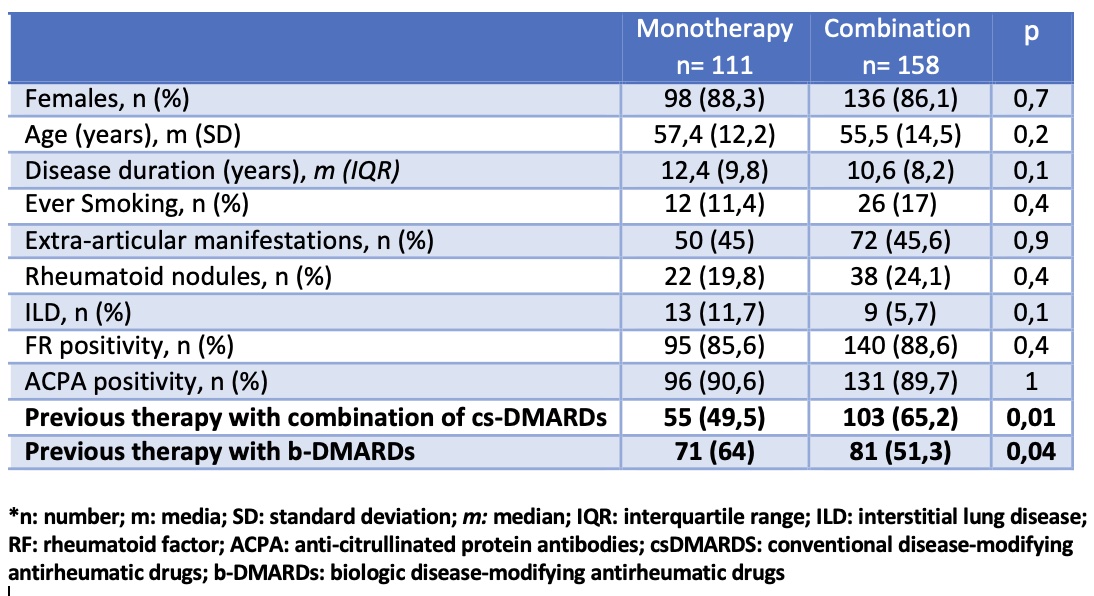
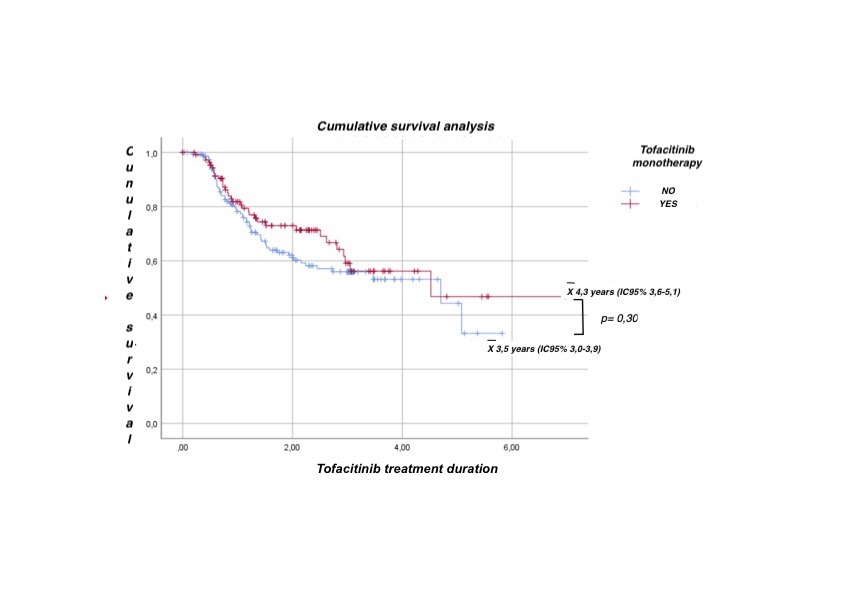
Results: 269 patients were included, 87% female, mean age 56.3 years (SD 13.6), of which 41.3% started treatment with tofacitinib monotherapy. Main reasons for monotherapy use were previous adverse events with csDMARD (16%) and physician's decision (16%). In the univariate analysis, no significant association was found between the use of monotherapy and different sociodemographic and clinical variables. In the multivariate analysis, fewer prior cs-DMARDs combination therapy and prior use of biologic therapy were significantly associated with initiation of tofacitinib monotherapy (p< 0.05, both) (table). During follow-up (502.4 patients/year), no statistically significant differences were found in tofacitinib survival between the two groups (figure). Regarding safety, 110 patients (40.9%) had at least 1 AE, the most frequent being infections (57.6%), others (16.7%), and gastrointestinal (9.5%). When evaluating the incidence rate (IR) of AEs, no significant differences were found between tofacitinib monotherapy and combination therapy: 47.2 events (100 p/y) (95% CI 38.6-57.5) vs. 38.05 events (100 p/y) (95%CI 31.6-45.7), respectively (p=NS).
Conclusion: In this real-life study, the use of tofacitinib monotherapy was almost 40%, being associated with less prior treatment with csDMARDs and prior use of biologic therapy. Tofacitinib monotherapy demonstrated similar drug survival and safety compared to the use in combination with csDMARDs, and may be a valid option for patients with RA in daily clinical practice.
R. Perez-Alamino: None; C. Isnardi: None; E. Soriano: AbbVie, 2, 5, 6, Amgen, 6, Bristol-Myers Squibb, 6, Eli Lilly, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 5, 6, Roche, 2, 5, 6, UCB, 5, 6; L. Lo Giudice: None; j. zacariaz hereter: None; G. Casado: None; V. Caputo: None; A. Schmichowski: None; C. Romeo: None; E. Rivero: None; F. Savy: None; M. García: GSK, 6, Janssen, 6, Pfizer, 6; O. Romano: None; H. Maldonado Ficco: None; G. Citera: None.
Background/Purpose: Different guidelines recommend the use of tofacitinib either in monotherapy or combined with methotrexate (combination therapy) in patients with rheumatoid arthritis (RA) with an inadequate response to conventional synthetic DMARDs (csDMARDs) or biologic DMARDs (bDMARDs). Although the evidence originating from clinical trials does not show notable differences in terms of efficacy and safety with both strategies, data from real life is limited.
To evaluate the frequency, predictors of use and survival of tofacitinib in monotherapy compared with combination therapy in a cohort of patients with RA from daily clinical practice in our country.
Methods: Longitudinal, observational study which includes a cohort of patients with RA (ACR/EULAR 2010 criteria) from different rheumatology centers who started treatment with tofacitinib. Patients were evaluated at baseline and followed up every six months from the start of medication. Sociodemographic, clinical and treatment data were collected. Chi2 test, Fisher's exact test, Student's T test and Mann Whitney were used according to the nature of the variables. Kaplan-Meier method and the Log Rank test were applied to compare survival of treatment in monotherapy vs. combination therapy. Cox regression analysis was used to evaluate variables associated with lower survival. The incidence of adverse events (AEs) was calculated as events every 100 patients/year (p/y). A p< 0.05 was considered significant.
Results: 269 patients were included, 87% female, mean age 56.3 years (SD 13.6), of which 41.3% started treatment with tofacitinib monotherapy. Main reasons for monotherapy use were previous adverse events with csDMARD (16%) and physician's decision (16%). In the univariate analysis, no significant association was found between the use of monotherapy and different sociodemographic and clinical variables. In the multivariate analysis, fewer prior cs-DMARDs combination therapy and prior use of biologic therapy were significantly associated with initiation of tofacitinib monotherapy (p< 0.05, both) (table). During follow-up (502.4 patients/year), no statistically significant differences were found in tofacitinib survival between the two groups (figure). Regarding safety, 110 patients (40.9%) had at least 1 AE, the most frequent being infections (57.6%), others (16.7%), and gastrointestinal (9.5%). When evaluating the incidence rate (IR) of AEs, no significant differences were found between tofacitinib monotherapy and combination therapy: 47.2 events (100 p/y) (95% CI 38.6-57.5) vs. 38.05 events (100 p/y) (95%CI 31.6-45.7), respectively (p=NS).
Conclusion: In this real-life study, the use of tofacitinib monotherapy was almost 40%, being associated with less prior treatment with csDMARDs and prior use of biologic therapy. Tofacitinib monotherapy demonstrated similar drug survival and safety compared to the use in combination with csDMARDs, and may be a valid option for patients with RA in daily clinical practice.

Predictors factors of initiation of treatment with tofacitinib monotherapy

Cumulative survival analysis between tofacitinib monotherapy vs. combination therapy
R. Perez-Alamino: None; C. Isnardi: None; E. Soriano: AbbVie, 2, 5, 6, Amgen, 6, Bristol-Myers Squibb, 6, Eli Lilly, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 5, 6, Roche, 2, 5, 6, UCB, 5, 6; L. Lo Giudice: None; j. zacariaz hereter: None; G. Casado: None; V. Caputo: None; A. Schmichowski: None; C. Romeo: None; E. Rivero: None; F. Savy: None; M. García: GSK, 6, Janssen, 6, Pfizer, 6; O. Romano: None; H. Maldonado Ficco: None; G. Citera: None.



