Abstract Session
Periodic fever syndromes, autoinflammatory diseases, Still’s disease and MAS/HLH
Session: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases II (2515–2520)
2519: A Phase II Clinical Study to Investigate the Efficacy and Safety of Hemay005 Tablets in Patients with Active Behçet`sDisease
Tuesday, November 14, 2023
5:00 PM - 5:10 PM PT
Location: Room 6E/6D
- CJ
Charles Jones, DM, MRCP (he/him/his)
Hemay Pharmaceuticals
Naples, FL, United StatesDisclosure information not submitted.
Presenting Author(s)
Charles Jones1, Zhanguo Li2, Zhuoli Zhang3, Jian Wu4, Guixiu Shi5, Wenjie Zheng6, Jianping Tang7, Xiaobing Wang8, Lie Dai9, Lin Chen10, Yasong Li11, Ling Wu12, Yongfu Wang13, Shengyun Liu14, Yao Ke15, Jin Lin16, Zhenchun Zhang17, Jiankang Hu18, Wantai Dang19, Songlou Yin20, Xin Tang21, Mingfei Zhu22, Jinfeng Lin22, Richard Jones22, Weiguo Wan23 and xianjun Hu22, 1Hemay Pharmaceuticals, Tianjin, China, 2Peking University Health Science Center, Department of Rheumatology and Immunology, People's Hospital, Beijing, China, 3Peking University First Hospital, Rheumatology and Immunology Department, Beijing, China, 4Suzhou University Affiliated First Hospital, Suzhou, China, 5the First Affiliated Hospital of Xiamen University, Xiamen, China, 6Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Beijing, China, 7Shanghai Tongji Hospital, Shanghai, China, 8The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China, 9Department of Rheumatology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China, 10Jilin Provincial People's Hospital, Changchun, China, 1111Zhejiang Provincial People's Hospital, Hangzhou, China, 12University of Hong Kong Shenzhen Hospital, Shenzhen, China, 13The First Affiliated Hospital of Baotou Medical College, Baotou, China, 14The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 15Jiangsu Provincial People's Hospital, Nanjing, China, 16The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China, 17Linyi People's Hospital, Linyi, China, 18Pingxiang People’s Hospital, PingXiang, China, 19The First Affiliated Hospital of Chengdu Medical College, Chengdu, China, 20Xuzhou Medical University Affiliated Hospital, Xuzhou, China, 21Bestudy (Shanghai) Medical Technology Co., Ltd, Shanghai, China, 22Clinical Department, Tianjin Hemay Pharmaceutical Co., Ltd., Tianjin, China, 23Huashan Hospital Affiliated to Fudan University, Shanghai, China
Background/Purpose: Behçet's disease (BD) is a chronic and recurrent vascular inflammatory disease with major manifestations including oral ulcers, genital ulcers, skin damage and ophthalmitis, and it can also affect the nervous system, gastrointestinal tract, cardiovascular system, joints, and other vital organs. PDE IV inhibition is an approved therapy for BD. Hemay005 is a novel PDE IV inhibitor for treating chronic inflammatory diseases. Hemay005 significantly inhibits the activation of T lymphocytes, which play a vital role in the pathogenesis of Bechet's disease. It also inhibits Th1 type pro-inflammatory cytokines TNF-α, IFN-γ, IL-2, IL-12, and IL-23. Improvements in the side effect /efficacy ratio vs Apremilast are anticipated to improve on the efficacy of Apremilast, which was side effect limited at doses >30mg.
Methods: This was a multi-centre, randomized, double-blind, placebo-parallel-controlled, phase II clinical study. The study included four periods: a screening period, a 12-week core treatment period, a 12-week extension treatment period, and an off-drug observation period. All subjects completed the extension treatment period, followed by a 4-week off-drug observation period. It was planned to enrol a total of 252 patients, with randomization at 2:2:1:1 ratio. The study was terminated early as the efficacy objective was achieved after 90 patients were recruited. The Area under the curve (AUC) for the number of oral ulcers in BD patients from baseline to Week 12 was the primary efficacy endpoint. Adverse Events were also recorded as primary safety endpoint.
Results: Overall, 90 subjects were recruited; 29 subjects were assigned to Hemay005 tablet 45 mg BID group, 31 to Hemay005 tablet 60 mg BID group, and 30 to placebo group. Results based on the FAS showed that the Hemay005 60 mg BID (1-sided, P <0.0001) and 45 mg BID (1-sided, P <0.0001) were statistically different from placebo in reducing the AUCs for the number of oral ulcers from baseline to Week 12. Analysis of the median healing time of oral ulcer estimated by KM was 16 days in 45 mg BID group, with 95% CI (8, 46); 15 days in 60 mg BID group, with 95% CI (9, 29); and the median healing time could not be estimated by KM method in placebo group Fig 1.
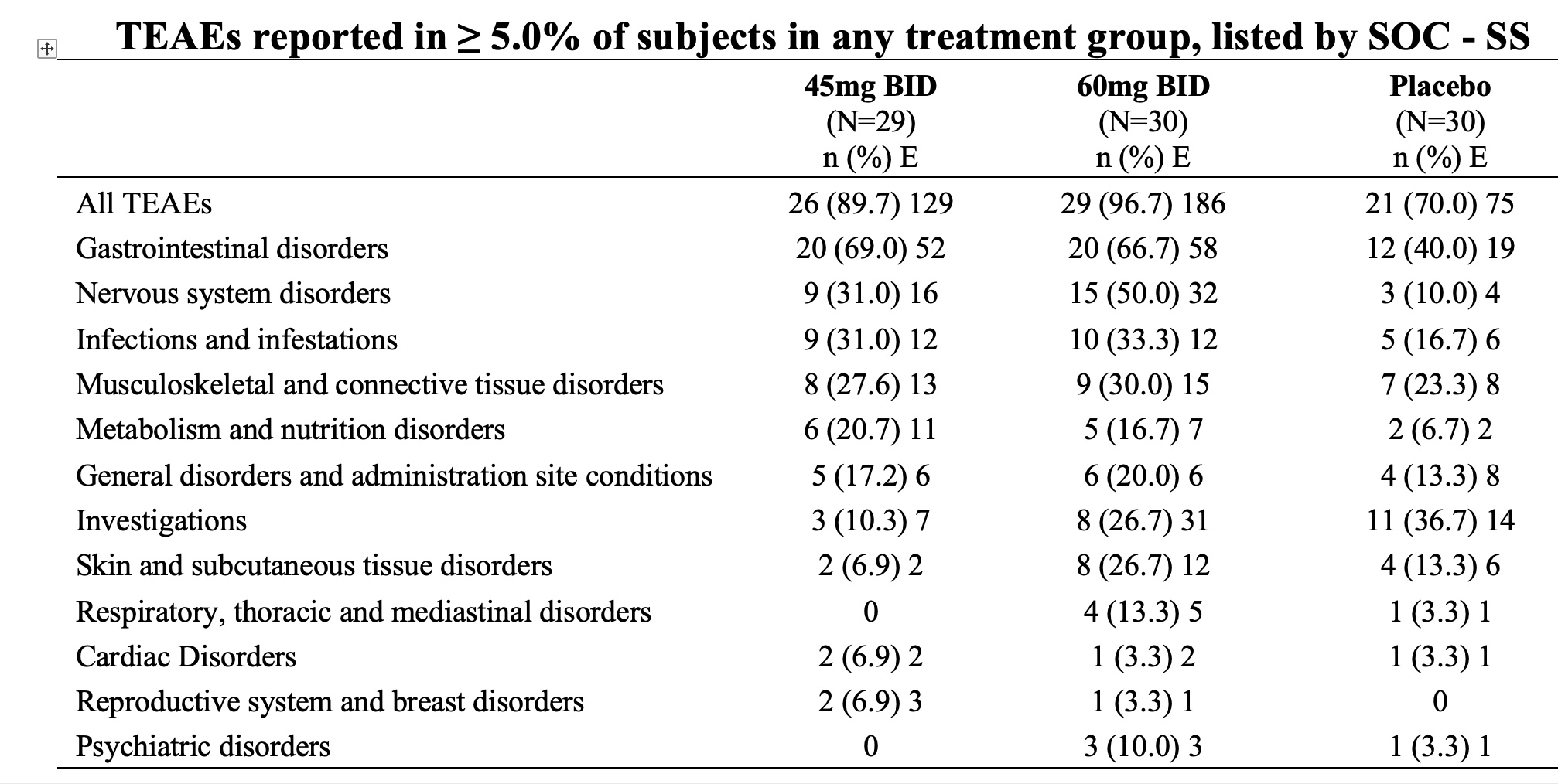
In the safety data set; (patients who received one dose or more of the drug or placebo), the incidence of TEAEs related to the study drug and leading to discontinuation of medication were higher in the 45 mg BID ( 6.9%) and 60 mg BID (6.7%)groups than that in the placebo group (0.0%) during the core treatment period. Most TEAEs reported during the core treatment period were mild; the incidence of severe TEAEs was dose-related, 3.3%, in the 60 mg BID and 0% in the 45 mg BID and placebo groups, respectively. No SAEs related to drug were reported throughout the study.
Conclusion: Hemay005 60 mg BID and 45 mg BID for 12 weeks effectively reduced the AUC for the number of oral ulcers from baseline to Week 12 in BD patients. The study drug was safe and well-tolerated. A phase Ⅲ clinical trial in patients with Bechet's disease is underway.
.jpg)
C. Jones: Hemay Pharmaceutical, 3; Z. Li: Abbott, 2, 9, Abbvie, 2, 9, BMS, 2, 9, Celgene, 2, 9, Eli Lilly, 2, 9, GSK, 2, 9, Janssen, 2, 9, MSD, 2, 9, Novartis, 2, 9, Pfizer, 2, 9, Roche, 2, 9, UCB Pharma, 2, 9; Z. Zhang: None; J. Wu: None; G. Shi: None; W. Zheng: None; J. Tang: None; X. Wang: None; L. Dai: None; L. Chen: None; Y. Li: None; L. Wu: None; Y. Wang: None; S. Liu: None; Y. Ke: None; J. Lin: None; Z. Zhang: None; J. Hu: None; W. Dang: None; S. Yin: None; X. Tang: None; M. Zhu: Hemay Pharmaceutical, 3; J. Lin: Hemay Pharmaceutical, 3; R. Jones: Hemay Pharmaceutical, 3; W. Wan: None; x. Hu: Hemay Pharmaceutical, 3.
Background/Purpose: Behçet's disease (BD) is a chronic and recurrent vascular inflammatory disease with major manifestations including oral ulcers, genital ulcers, skin damage and ophthalmitis, and it can also affect the nervous system, gastrointestinal tract, cardiovascular system, joints, and other vital organs. PDE IV inhibition is an approved therapy for BD. Hemay005 is a novel PDE IV inhibitor for treating chronic inflammatory diseases. Hemay005 significantly inhibits the activation of T lymphocytes, which play a vital role in the pathogenesis of Bechet's disease. It also inhibits Th1 type pro-inflammatory cytokines TNF-α, IFN-γ, IL-2, IL-12, and IL-23. Improvements in the side effect /efficacy ratio vs Apremilast are anticipated to improve on the efficacy of Apremilast, which was side effect limited at doses >30mg.
Methods: This was a multi-centre, randomized, double-blind, placebo-parallel-controlled, phase II clinical study. The study included four periods: a screening period, a 12-week core treatment period, a 12-week extension treatment period, and an off-drug observation period. All subjects completed the extension treatment period, followed by a 4-week off-drug observation period. It was planned to enrol a total of 252 patients, with randomization at 2:2:1:1 ratio. The study was terminated early as the efficacy objective was achieved after 90 patients were recruited. The Area under the curve (AUC) for the number of oral ulcers in BD patients from baseline to Week 12 was the primary efficacy endpoint. Adverse Events were also recorded as primary safety endpoint.
Results: Overall, 90 subjects were recruited; 29 subjects were assigned to Hemay005 tablet 45 mg BID group, 31 to Hemay005 tablet 60 mg BID group, and 30 to placebo group. Results based on the FAS showed that the Hemay005 60 mg BID (1-sided, P <0.0001) and 45 mg BID (1-sided, P <0.0001) were statistically different from placebo in reducing the AUCs for the number of oral ulcers from baseline to Week 12. Analysis of the median healing time of oral ulcer estimated by KM was 16 days in 45 mg BID group, with 95% CI (8, 46); 15 days in 60 mg BID group, with 95% CI (9, 29); and the median healing time could not be estimated by KM method in placebo group Fig 1.
In the safety data set; (patients who received one dose or more of the drug or placebo), the incidence of TEAEs related to the study drug and leading to discontinuation of medication were higher in the 45 mg BID ( 6.9%) and 60 mg BID (6.7%)groups than that in the placebo group (0.0%) during the core treatment period. Most TEAEs reported during the core treatment period were mild; the incidence of severe TEAEs was dose-related, 3.3%, in the 60 mg BID and 0% in the 45 mg BID and placebo groups, respectively. No SAEs related to drug were reported throughout the study.
Conclusion: Hemay005 60 mg BID and 45 mg BID for 12 weeks effectively reduced the AUC for the number of oral ulcers from baseline to Week 12 in BD patients. The study drug was safe and well-tolerated. A phase Ⅲ clinical trial in patients with Bechet's disease is underway.
.jpg)
Fig 1: Kaplan Meier curves: The time to first complete remission of oral ulcers -FAS

Table1 : The number of TEAS (MedDRA v 25); Percentages were calculated based on the number of subjects in the SS(Safety data set)
C. Jones: Hemay Pharmaceutical, 3; Z. Li: Abbott, 2, 9, Abbvie, 2, 9, BMS, 2, 9, Celgene, 2, 9, Eli Lilly, 2, 9, GSK, 2, 9, Janssen, 2, 9, MSD, 2, 9, Novartis, 2, 9, Pfizer, 2, 9, Roche, 2, 9, UCB Pharma, 2, 9; Z. Zhang: None; J. Wu: None; G. Shi: None; W. Zheng: None; J. Tang: None; X. Wang: None; L. Dai: None; L. Chen: None; Y. Li: None; L. Wu: None; Y. Wang: None; S. Liu: None; Y. Ke: None; J. Lin: None; Z. Zhang: None; J. Hu: None; W. Dang: None; S. Yin: None; X. Tang: None; M. Zhu: Hemay Pharmaceutical, 3; J. Lin: Hemay Pharmaceutical, 3; R. Jones: Hemay Pharmaceutical, 3; W. Wan: None; x. Hu: Hemay Pharmaceutical, 3.



