Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0543–0581) SLE – Diagnosis, Manifestations, & Outcomes Poster I
0552: The Impact of Antiphospholipid Antibodies on Future Atherosclerotic Cardiovascular Disease Risk in Systemic Lupus Erythematosus
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- YD
Yu Fang Ding, BS (she/her/hers)
Peking Union Medical College
BEIJING, Beijing, ChinaDisclosure information not submitted.
Abstract Poster Presenter(s)
YuFang Ding1, Can Huang2, Jiuliang zhao3, Qian Wang1, Xinping Tian3, Mengtao Li3 and xiaofeng Zeng1, 1Department of Rheumatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China, 2Peking Unoin Medical College Hospital, Beijing, China, 3Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Background/Purpose: Patients with systemic lupus erythematosus (SLE) suffered from an increasing risk of cardiovascular diseases (23·3 events per 1000 patient-years). Antiphospholipid antibodies (aPLs), including anticardiolipin antibodies (aCL), anti-𝛽2 glycoprotein I antibodies (aβ2GPI), and lupus anticoagulant (LA), increase the risk of thrombotic events in antiphospholipid syndrome, but the impact of aPLs on SLE patients was not yet determined. In this multi-center prospective study, we aimed to determine the association between aPLs and future atherosclerotic cardiovascular disease (ASCVD) risk in SLE.
Methods: Seven aPL isotypes (aCL IgG/IgM/IgA, aβ2GPI IgG/IgM/IgA, and LA) were measured based on international guidelines at SLE diagnosis and during follow-up. Clinical manifestations, disease activity status and organ damage were collected. Future ASCVD events were defined as new onset nonfatal myocardial infarction, nonfatal stroke, coronary or peripheral artery revascularization, or cardiovascular death.
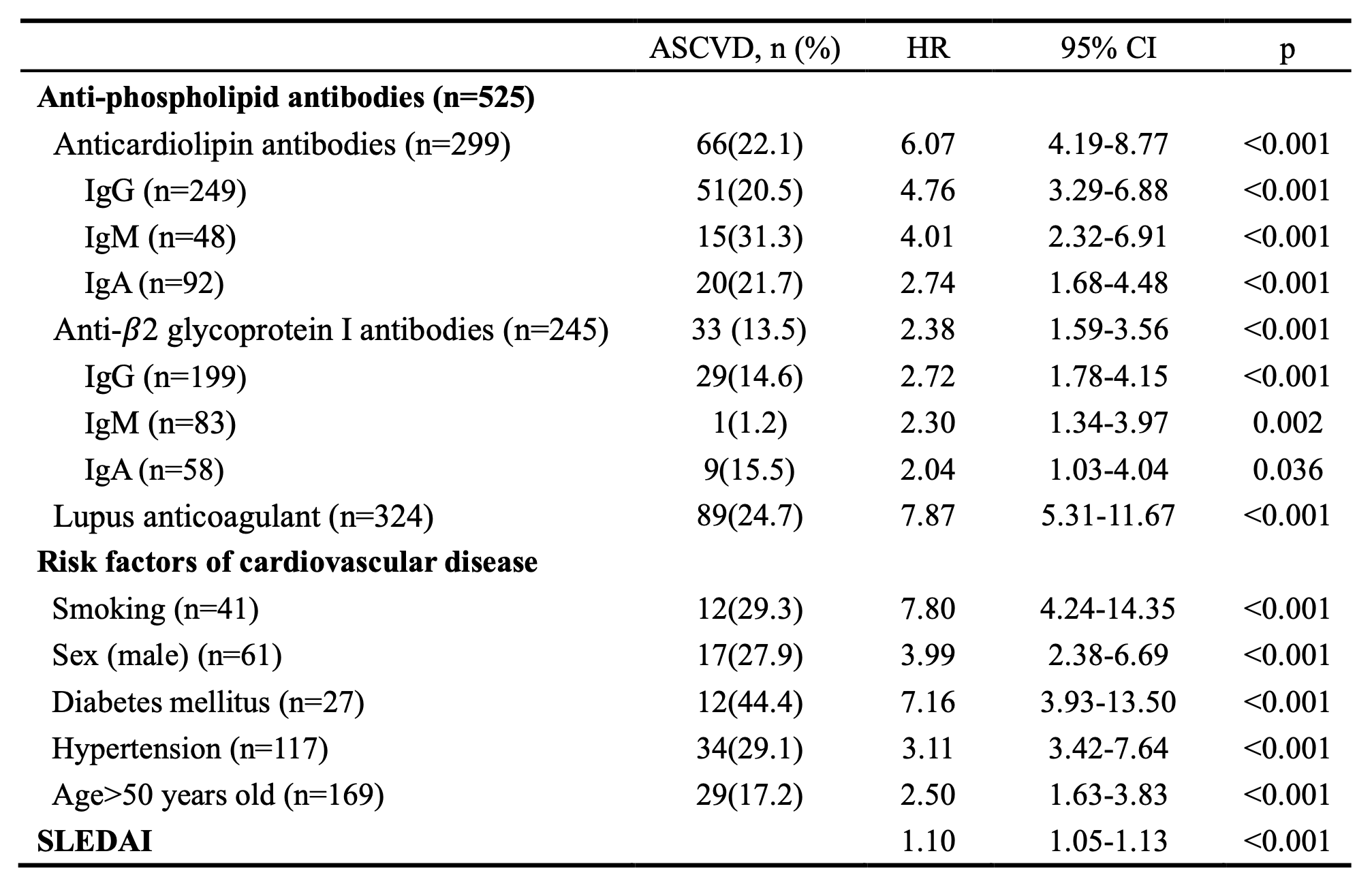
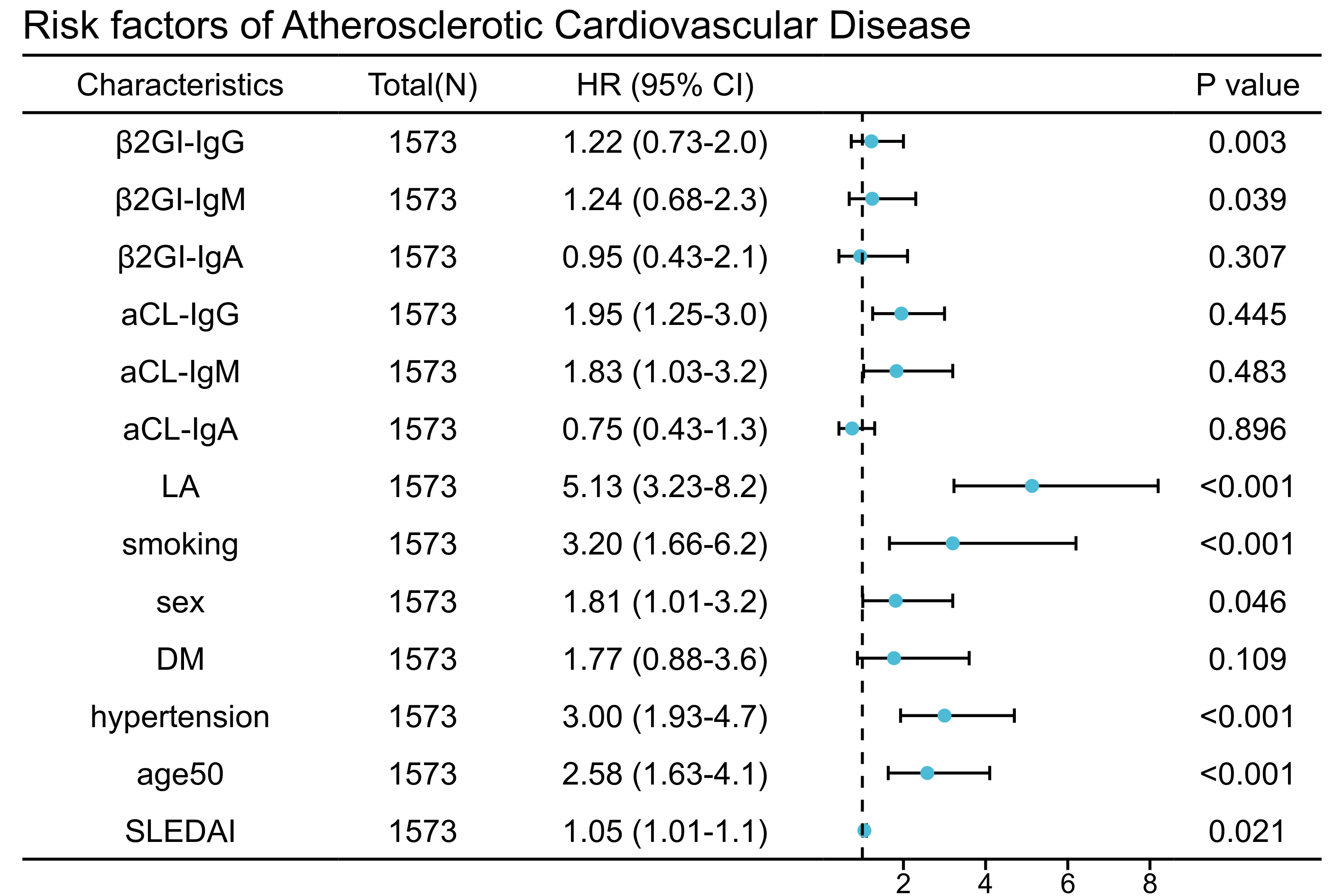
Results: Among the 1573 recruited SLE patients, 525 (33.4%) had positive aPLs. LA had the highest prevalence (324 [20.6%]), followed by aCL IgG (249 [15.8%]), aβ2GPI IgG (199 [12.7%]), aCL IgA (58 [3.7%]), aβ2GPI IgM (83 [5.3%]), aβ2GPI IgA (58 [3.7%]), aCL IgM (92 [5.8%]). 116 (7.37%) patients developed ASCVD during the mean follow-up of 4.51±2.32 years and 92 patients were aPLs positive. In univariate Cox regression analysis, both aPLs (HR=7.81, 95% CI, 5.00-12.24, p< 0.001) and traditional risk factors of cardiovascular disease were associated with future ASCVD events (Table 1). Kaplan-Meier plots suggested a potential trend towards ASCVD in positive versus negative aPLs antibodies. More importantly, anticoagulant or antiplatelet therapy can reduce ASCVD risk in SLE patients with positive aPLs (HR=0.57, 95% CI,0.25-0.93, P=0.026) (Figure 1). In multiple Cox regression analysis, aCL IgG (HR=1.95, 95% CI, 1.25-3.00, p=0.003), aCL IgM (HR=1.83, 95% CI, 1.03-3.20, p=0.039), and LA (HR=5.13, 95% CI 3.23-8.20, p< 0.001) positivity remained independently associated with ASCVD; traditional risk factors for ASCVD, including smoking, gender, age and hypertension, also play an independent role in SLE patients (Figure 2).
Conclusion: SLE patients with positive aPLs, especially positive aCL IgG/IgM and LA, warrant more care and surveillance of future ASCVD events during follow-up. Antithrombotic therapy has a protective effect on future ASCVD.
.jpg)
Y. Ding: None; C. Huang: None; J. zhao: None; Q. Wang: None; X. Tian: None; M. Li: None; x. Zeng: None.
Background/Purpose: Patients with systemic lupus erythematosus (SLE) suffered from an increasing risk of cardiovascular diseases (23·3 events per 1000 patient-years). Antiphospholipid antibodies (aPLs), including anticardiolipin antibodies (aCL), anti-𝛽2 glycoprotein I antibodies (aβ2GPI), and lupus anticoagulant (LA), increase the risk of thrombotic events in antiphospholipid syndrome, but the impact of aPLs on SLE patients was not yet determined. In this multi-center prospective study, we aimed to determine the association between aPLs and future atherosclerotic cardiovascular disease (ASCVD) risk in SLE.
Methods: Seven aPL isotypes (aCL IgG/IgM/IgA, aβ2GPI IgG/IgM/IgA, and LA) were measured based on international guidelines at SLE diagnosis and during follow-up. Clinical manifestations, disease activity status and organ damage were collected. Future ASCVD events were defined as new onset nonfatal myocardial infarction, nonfatal stroke, coronary or peripheral artery revascularization, or cardiovascular death.
Results: Among the 1573 recruited SLE patients, 525 (33.4%) had positive aPLs. LA had the highest prevalence (324 [20.6%]), followed by aCL IgG (249 [15.8%]), aβ2GPI IgG (199 [12.7%]), aCL IgA (58 [3.7%]), aβ2GPI IgM (83 [5.3%]), aβ2GPI IgA (58 [3.7%]), aCL IgM (92 [5.8%]). 116 (7.37%) patients developed ASCVD during the mean follow-up of 4.51±2.32 years and 92 patients were aPLs positive. In univariate Cox regression analysis, both aPLs (HR=7.81, 95% CI, 5.00-12.24, p< 0.001) and traditional risk factors of cardiovascular disease were associated with future ASCVD events (Table 1). Kaplan-Meier plots suggested a potential trend towards ASCVD in positive versus negative aPLs antibodies. More importantly, anticoagulant or antiplatelet therapy can reduce ASCVD risk in SLE patients with positive aPLs (HR=0.57, 95% CI,0.25-0.93, P=0.026) (Figure 1). In multiple Cox regression analysis, aCL IgG (HR=1.95, 95% CI, 1.25-3.00, p=0.003), aCL IgM (HR=1.83, 95% CI, 1.03-3.20, p=0.039), and LA (HR=5.13, 95% CI 3.23-8.20, p< 0.001) positivity remained independently associated with ASCVD; traditional risk factors for ASCVD, including smoking, gender, age and hypertension, also play an independent role in SLE patients (Figure 2).
Conclusion: SLE patients with positive aPLs, especially positive aCL IgG/IgM and LA, warrant more care and surveillance of future ASCVD events during follow-up. Antithrombotic therapy has a protective effect on future ASCVD.

Table 1. Proportion of ASCVD in SLE patients with different isotypes of aPLs
.jpg)
Figure 1. Cumulative probability of ASCVD in patients with or without aPLs (A), aβ2-GPI IgG antibody (B), aβ2-GPI IgM antibody (C), aβ2-GPI IgA antibody (D), aCL IgG antibody (E), aCL IgM antibody (F), aCL IgA antibody (G), and LA (H). (I) Cumulative probability of ASCVD in aPLs negative patients, aPLs positive patients with anticoagulant or antiplatelet therapy, and aPLs positive patients without anticoagulant or antiplatelet therapy.
Figure 2. Risk factors of ASCVD in SLE patients.
Y. Ding: None; C. Huang: None; J. zhao: None; Q. Wang: None; X. Tian: None; M. Li: None; x. Zeng: None.



