Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes III: Disease Activity (2551–2556)
2554: Validation of a Flare Risk Index Informed by Select Immune Mediators in Systemic Lupus Erythematosus in a Confirmatory Cohort
Tuesday, November 14, 2023
4:45 PM - 4:55 PM PT
Location: Ballroom 20A
.png)
Melissa Munroe, MD, PhD (she/her/hers)
Oklahoma Medical Research Foundation
Oklahoma City, OK, United StatesDisclosure information not submitted.
Presenting Author(s)
Melissa Munroe1, Derek Blankenship2, Daniele DeFreese2, Adrian Holloway2, Mohan Purushothaman2, Wade DeJager3, Susan Macwana3, Joel Guthridge3, Stan Kamp3, Nancy Redinger3, Teresa Aberle3, Eliza Chakravarty3, Cristina Arriens4, Yanfeng Li5, Hu Zeng5, Stephanie Dezzutti6, Peter Izmirly7, Uma Thanarajasingam5, Diane L. Kamen6, Jill Buyon8, Judith James3 and Eldon Jupe2, 1Oklahoma Medical Research Foundation; Progentec Diagnostics, Inc., Oklahoma City, OK, 2Progentec Diagnostics, Inc., Oklahoma City, OK, 3Oklahoma Medical Research Foundation, Oklahoma City, OK, 4Oklahoma Medical Research Foundation and University of Oklahoma Health Sciences Center, Department of Arthritis & Clinical Immunology, Oklahoma City, OK, 5Mayo Clinic, Rochester, MN, 6Medical University of South Carolina, Charleston, SC, 7New York University School of Medicine, New York, NY, 8NYU Grossman School of Medicine, New York, NY
Background/Purpose: SLE is marked by immune dysregulation linked to varied clinical disease activity. Using a unique confirmatory cohort of SLE patients, this study seeks to validate a recently refined Lupus Flare Risk Index (L-FRI; Munroe et al. Arthritis Rheumatol. 2023) reflecting altered immunity prior to clinical disease flare.
Methods: The L-FRI is the sum of log-transformed, standardized immune mediators, weighted by the Spearman r correlation coefficient for each preflare/prenonflare analyte vs. subsequent flare/nonflare hSLEDAI disease activity (flare is defined by the SELENA-SLEDAI Flare Index). SLE-associated plasma mediators (n=11) were evaluated by microfluidic immunoassay in 52 preflare and 52 prenonflare samples from patients with classified SLE. Hybrid SLEDAI (hSLEDAI) scores, clinical features, medication usage, and the presence of SLE-associated autoantibody (AutoAb) specificities, including dsDNA, chromatin, Ro/SSA, La/SSB, Sm, SmRNP, and RNP, were also compared at preflare (105 ± 62 days prior to flare) vs. prenonflare (105 ± 55 days prior to nonflare) time points. Data from this validation cohort were compared to development and merged development/validation cohorts.
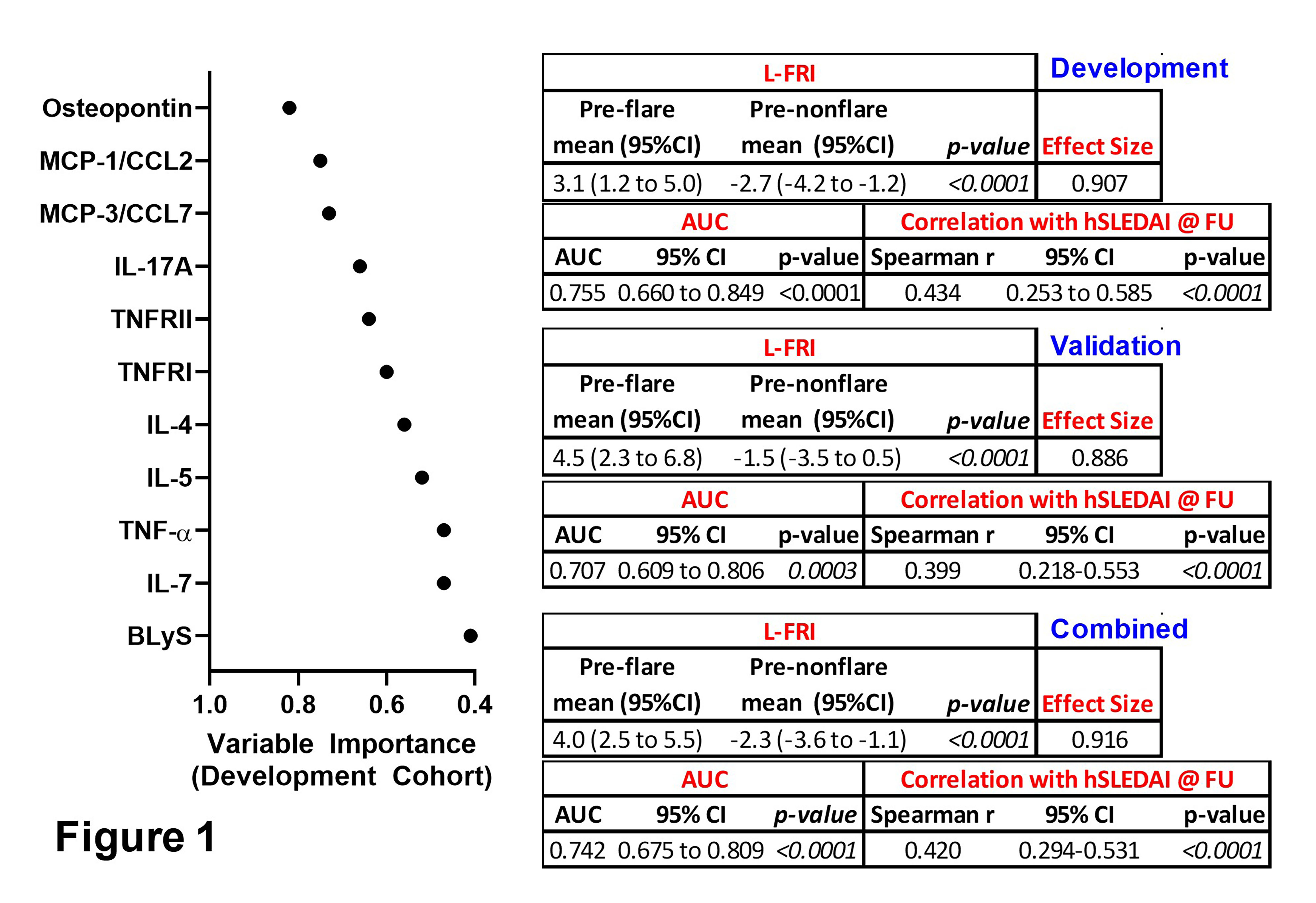
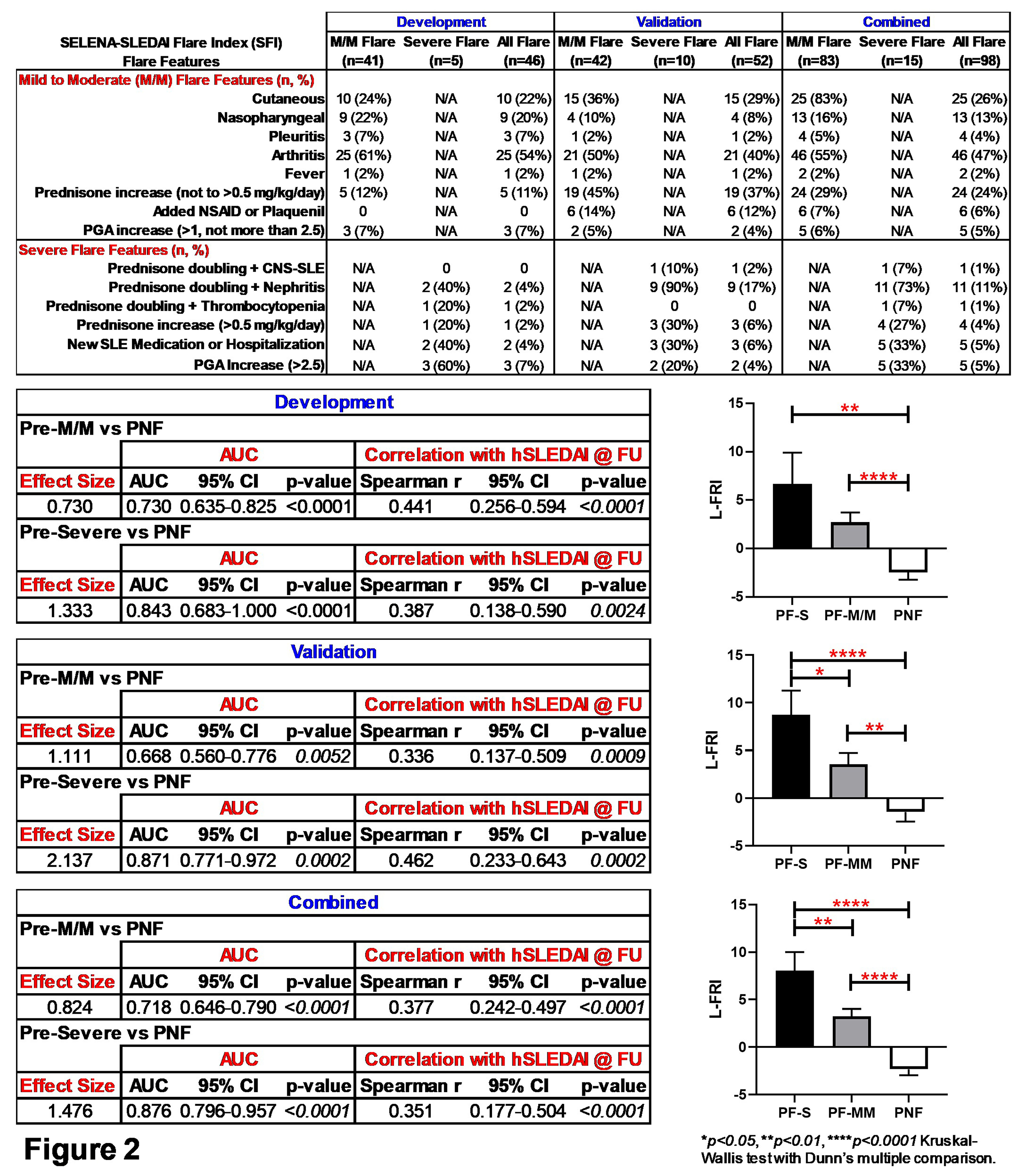
Results: This validation (Val) cohort is enriched for African American (AA) SLE patients (44% preflare, 48% prenonflare vs. 11% preflare, 15% prenonflare in the development [Dev] cohort), with an associated increase in preflare hSLEDAI scores (4.0±3.4 preflare, 1.9±2.2 prenonflare, p=0.0004 [Val] vs. 2.4±2.6 preflare, 2.8±3.8 prenonflare, p=0.8953 [Dev]). Otherwise, after adjusting for multiple comparison, we did not observe differences with respect to clinical features, medication usage, or number and type of SLE-associated AutoAbs in preflare vs. prenonflare samples. The L-FRI, informed by 11 mediators (Fig. 1), significantly (p< 0.0001) differentiated preflare vs. prenonflare samples in the Val cohort, similar to that of the Dev and combined (Com) cohorts (Fig. 1), with a large ( >0.8) Cohen's effect size, AUC >0.7 (p≤0.0003), and Spearman r ≥0.399 (p< 0.0001) vs. hSLEDAI scores at disease flare/nonflare (Fig. 1). The inclusion of 11 mediators in the L-FRI allows for varied differences between preflare vs. prenonflare across cohorts, yet with consistent differences in osteopontin, TNFRII, TNFRI, and TNF-α (Table 1). Furthermore, the L-FRI differentiates SLE patients at risk of imminent severe (S) and mild-moderate (M/M) flares vs. nonflare (Fig. 2), with increased L-FRI scores (p≤0.05), effect size (≥1.3), and AUC (≥0.843, p≤0.0002) in pre-severe flare samples across the Val, Dev, and Com cohorts (Fig. 2).
Conclusion: We verified the utility of the L-FRI, informed by 11 immune mediators, to identify SLE patients at risk of imminent lupus disease flare. Of particular interest is the ability of the L-FRI to differentiate future M/M vs. severe flare risk. A subset of mediators consistently enhanced the L-FRI to identify SLE patients who may benefit from early intervention strategies. Such an approach would be advantageous in prospective clinical trials for study participant recruitment and assessment, as well as improved management of lupus
.jpg)
M. Munroe: Progentec Diagnostics, Inc., 12, Salary Support; D. Blankenship: Progentec Diagnostics, Inc., 2; D. DeFreese: Progentec Diagnostics, Inc., 3; A. Holloway: Progentec Diagnostics, Inc., 3; M. Purushothaman: Progentec Diagnostics, Inc., 3, 4; W. DeJager: None; S. Macwana: None; J. Guthridge: None; S. Kamp: None; N. Redinger: None; T. Aberle: None; E. Chakravarty: None; C. Arriens: AstraZeneca, 1, 5, 6, Aurinia, 6, Bristol-Myers Squibb, 1, 5, Cabaletta, 1, GSK, 1, Kezar, 1, UCB, 1; Y. Li: None; H. Zeng: None; S. Dezzutti: None; P. Izmirly: None; U. Thanarajasingam: None; D. Kamen: None; J. Buyon: Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, Related Sciences, 1; J. James: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 2, Novartis, 2, Progentec Biosciences, 5; E. Jupe: Progentec Diagnostics, Inc., 3.
Background/Purpose: SLE is marked by immune dysregulation linked to varied clinical disease activity. Using a unique confirmatory cohort of SLE patients, this study seeks to validate a recently refined Lupus Flare Risk Index (L-FRI; Munroe et al. Arthritis Rheumatol. 2023) reflecting altered immunity prior to clinical disease flare.
Methods: The L-FRI is the sum of log-transformed, standardized immune mediators, weighted by the Spearman r correlation coefficient for each preflare/prenonflare analyte vs. subsequent flare/nonflare hSLEDAI disease activity (flare is defined by the SELENA-SLEDAI Flare Index). SLE-associated plasma mediators (n=11) were evaluated by microfluidic immunoassay in 52 preflare and 52 prenonflare samples from patients with classified SLE. Hybrid SLEDAI (hSLEDAI) scores, clinical features, medication usage, and the presence of SLE-associated autoantibody (AutoAb) specificities, including dsDNA, chromatin, Ro/SSA, La/SSB, Sm, SmRNP, and RNP, were also compared at preflare (105 ± 62 days prior to flare) vs. prenonflare (105 ± 55 days prior to nonflare) time points. Data from this validation cohort were compared to development and merged development/validation cohorts.
Results: This validation (Val) cohort is enriched for African American (AA) SLE patients (44% preflare, 48% prenonflare vs. 11% preflare, 15% prenonflare in the development [Dev] cohort), with an associated increase in preflare hSLEDAI scores (4.0±3.4 preflare, 1.9±2.2 prenonflare, p=0.0004 [Val] vs. 2.4±2.6 preflare, 2.8±3.8 prenonflare, p=0.8953 [Dev]). Otherwise, after adjusting for multiple comparison, we did not observe differences with respect to clinical features, medication usage, or number and type of SLE-associated AutoAbs in preflare vs. prenonflare samples. The L-FRI, informed by 11 mediators (Fig. 1), significantly (p< 0.0001) differentiated preflare vs. prenonflare samples in the Val cohort, similar to that of the Dev and combined (Com) cohorts (Fig. 1), with a large ( >0.8) Cohen's effect size, AUC >0.7 (p≤0.0003), and Spearman r ≥0.399 (p< 0.0001) vs. hSLEDAI scores at disease flare/nonflare (Fig. 1). The inclusion of 11 mediators in the L-FRI allows for varied differences between preflare vs. prenonflare across cohorts, yet with consistent differences in osteopontin, TNFRII, TNFRI, and TNF-α (Table 1). Furthermore, the L-FRI differentiates SLE patients at risk of imminent severe (S) and mild-moderate (M/M) flares vs. nonflare (Fig. 2), with increased L-FRI scores (p≤0.05), effect size (≥1.3), and AUC (≥0.843, p≤0.0002) in pre-severe flare samples across the Val, Dev, and Com cohorts (Fig. 2).
Conclusion: We verified the utility of the L-FRI, informed by 11 immune mediators, to identify SLE patients at risk of imminent lupus disease flare. Of particular interest is the ability of the L-FRI to differentiate future M/M vs. severe flare risk. A subset of mediators consistently enhanced the L-FRI to identify SLE patients who may benefit from early intervention strategies. Such an approach would be advantageous in prospective clinical trials for study participant recruitment and assessment, as well as improved management of lupus

.jpg)

M. Munroe: Progentec Diagnostics, Inc., 12, Salary Support; D. Blankenship: Progentec Diagnostics, Inc., 2; D. DeFreese: Progentec Diagnostics, Inc., 3; A. Holloway: Progentec Diagnostics, Inc., 3; M. Purushothaman: Progentec Diagnostics, Inc., 3, 4; W. DeJager: None; S. Macwana: None; J. Guthridge: None; S. Kamp: None; N. Redinger: None; T. Aberle: None; E. Chakravarty: None; C. Arriens: AstraZeneca, 1, 5, 6, Aurinia, 6, Bristol-Myers Squibb, 1, 5, Cabaletta, 1, GSK, 1, Kezar, 1, UCB, 1; Y. Li: None; H. Zeng: None; S. Dezzutti: None; P. Izmirly: None; U. Thanarajasingam: None; D. Kamen: None; J. Buyon: Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, Related Sciences, 1; J. James: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 2, Novartis, 2, Progentec Biosciences, 5; E. Jupe: Progentec Diagnostics, Inc., 3.



