Poster Session A
Rheumatoid arthritis (RA)
Session: (0380–0422) RA – Diagnosis, Manifestations, and Outcomes Poster I
0387: The Association Between Inflammation, High-Sensitivity Cardiac Troponin T, and Cardiovascular Events in Rheumatoid Arthritis
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
.png)
Katherine Liao, MD, MPH
Brigham and Women's Hospital
Boston, MA, United StatesDisclosure(s): UCB: Consultant (Terminated, October 5, 2022)
Abstract Poster Presenter(s)
Brittany Weber1, Dana Weisenfeld1, Mary Jeffway1, Jonathan Coblyn1, Michael Weinblatt2, Nancy Shadick3, Marcelo DiCarli1 and Katherine Liao1, 1Brigham and Women's Hospital, Boston, MA, 2Harvard Medical School, Waban, MA, 3Brigham and Women's Hospital, Boston, MA
Background/Purpose: Patients with rheumatoid arthritis (RA) have a 1.5x excess risk of cardiovascular (CV) disease compared to the general population, attributed to chronic inflammation. In the general population, detectable levels of high sensitivity cardiac troponin (hs-cTnT) are associated with higher risk of major cardiovascular (CV) events (MACE); while this same relationship is expected in RA, we sought to determine whether inflammation may modify this relationship. The objective of this study was to test the association between hs-cTnT with MACE, and further determine whether this relationship may differ by inflammatory states.
Methods: We studied 185 patients in a longitudinal RA cohort with blood samples and hsCRP measured annually, who experienced either a significant increase in hsCRP low→high or decrease in hsCRP high→low, defined as hsCRP≥10 mg/L, in 2 consecutive annual visits. Subjects on statin therapy or lipid-lowering therapy were excluded. Hs-cTnT, a marker of subclinical myocardial injury and routine lipids were measured at baseline; estimated 10-year atherosclerotic CV risk was also calculated at baseline. The primary outcome, MACE was obtained using medical record review from linked electronic health record (EHR) data. Patients were followed from baseline until MACE, death, last EHR encounter or 15 years, whichever occurred first. The association between detectable hs-cTnT with MACE were determined using Cox proportional hazards models stratified by cohort status, i.e., hsCRP low→high cohort vs hsCRP high → low cohort; models were further adjusted be CV risk factors including age, sex, hypertension, hyperlipidemia, and smoking.
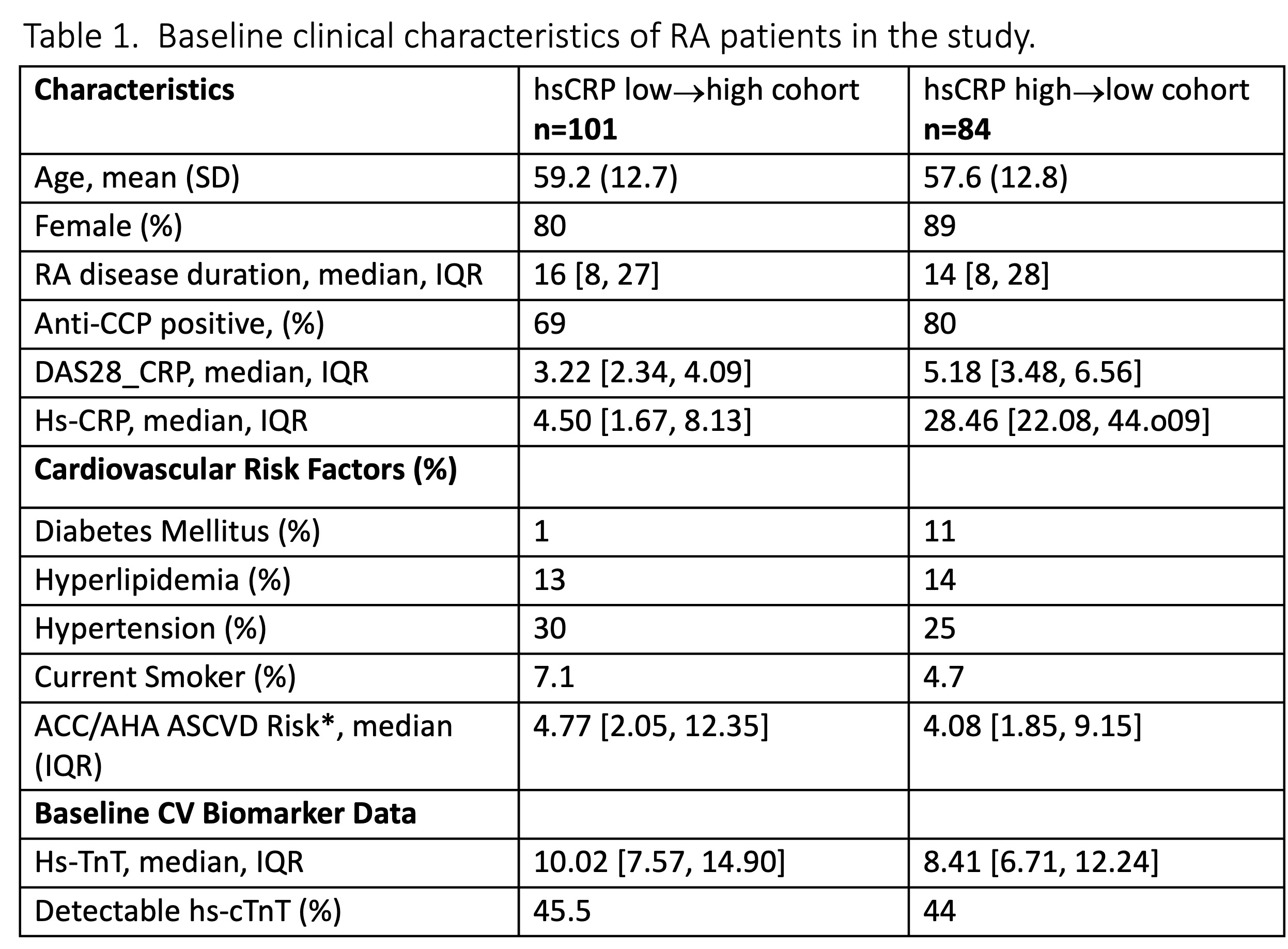
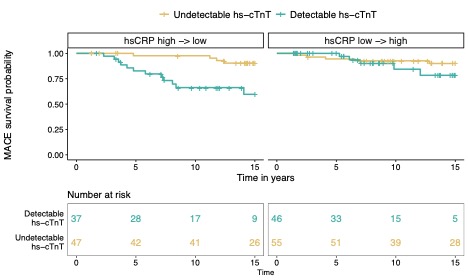
Results: The mean age was 58 years, 84% female, 74% anti-CCP positive with median RA disease duration of 15 years (Table 1). A total of 26 MACE events (14%) occurred during this period. The baseline median hsCRP among the hsCRP low→high cohort was 4.5 mg/dl [IQR 1.7-8.1) and in the hsCRP high→low cohort 28.5 mg/dl [IQR 22-44]. At baseline, in hsCRP low→high cohort, 46% had a detectable hs-cTnT, while 44% were detectable in the hsCRP high→low cohort. Detectable hs-cTnT at baseline was associated with future MACE HR 5.6 (95% CI 1.78-17.6; p=0.003) in the hsCRP high→low cohort with high inflammation at baseline; no association was observed with MACE in the hsCRP low→high cohort with low inflammation at baseline HR 1.84 (95% CI 0.53-6.41; p=0.3) (Figure 1). These associations remained after adjusting for CV risk factors.
Conclusion: In this RA cohort with overall low estimated ASCVD risk by standard CV risk scores, detectable hs-cTnT was associated with future MACE independent of traditional CV risk factors among those experiencing a high inflammatory state but not among those in a lower inflammatory state. These findings suggest that hs-cTnT may provide additional info for assessing CV risk among RA patients with overall low estimated ASCVD risk with active inflammation.
B. Weber: Bristol-Myers Squibb(BMS), 1, Horizon Therapeutics, 1, Kiniksa, 1, Novo Nordisk, 1; D. Weisenfeld: None; M. Jeffway: None; J. Coblyn: None; M. Weinblatt: Abbvie, 2, 5, Aclaris, 2, Amgen, 2, Aqtual, 5, Bristol Myers Squibb, 2, 5, Canfite, 11, Corevitas, 2, CorEvitas, 2, Eli Lilly, 2, Gilead, 2, 2, Glaxo Smith Kline, 2, Horizon, 2, Inmedix, 11, Janssen, 5, Johnson and Johnson, 2, Pfizer, 2, Prometheus Laboratories, 2, Rani, 2, Revolo, 2, Sanofi, 2, Sci Rhom, 2, Scipher, 2, 11, Set Point, 2, UCB, 2; N. Shadick: Abbvie, 5, AQtual, 5, Bristol-Myers Squibb(BMS), 5, Janssen, 5; M. DiCarli: Amgen, 2, Gilead, 5, Medtrace, 2, sanofi, 2; K. Liao: UCB, 2.
Background/Purpose: Patients with rheumatoid arthritis (RA) have a 1.5x excess risk of cardiovascular (CV) disease compared to the general population, attributed to chronic inflammation. In the general population, detectable levels of high sensitivity cardiac troponin (hs-cTnT) are associated with higher risk of major cardiovascular (CV) events (MACE); while this same relationship is expected in RA, we sought to determine whether inflammation may modify this relationship. The objective of this study was to test the association between hs-cTnT with MACE, and further determine whether this relationship may differ by inflammatory states.
Methods: We studied 185 patients in a longitudinal RA cohort with blood samples and hsCRP measured annually, who experienced either a significant increase in hsCRP low→high or decrease in hsCRP high→low, defined as hsCRP≥10 mg/L, in 2 consecutive annual visits. Subjects on statin therapy or lipid-lowering therapy were excluded. Hs-cTnT, a marker of subclinical myocardial injury and routine lipids were measured at baseline; estimated 10-year atherosclerotic CV risk was also calculated at baseline. The primary outcome, MACE was obtained using medical record review from linked electronic health record (EHR) data. Patients were followed from baseline until MACE, death, last EHR encounter or 15 years, whichever occurred first. The association between detectable hs-cTnT with MACE were determined using Cox proportional hazards models stratified by cohort status, i.e., hsCRP low→high cohort vs hsCRP high → low cohort; models were further adjusted be CV risk factors including age, sex, hypertension, hyperlipidemia, and smoking.
Results: The mean age was 58 years, 84% female, 74% anti-CCP positive with median RA disease duration of 15 years (Table 1). A total of 26 MACE events (14%) occurred during this period. The baseline median hsCRP among the hsCRP low→high cohort was 4.5 mg/dl [IQR 1.7-8.1) and in the hsCRP high→low cohort 28.5 mg/dl [IQR 22-44]. At baseline, in hsCRP low→high cohort, 46% had a detectable hs-cTnT, while 44% were detectable in the hsCRP high→low cohort. Detectable hs-cTnT at baseline was associated with future MACE HR 5.6 (95% CI 1.78-17.6; p=0.003) in the hsCRP high→low cohort with high inflammation at baseline; no association was observed with MACE in the hsCRP low→high cohort with low inflammation at baseline HR 1.84 (95% CI 0.53-6.41; p=0.3) (Figure 1). These associations remained after adjusting for CV risk factors.
Conclusion: In this RA cohort with overall low estimated ASCVD risk by standard CV risk scores, detectable hs-cTnT was associated with future MACE independent of traditional CV risk factors among those experiencing a high inflammatory state but not among those in a lower inflammatory state. These findings suggest that hs-cTnT may provide additional info for assessing CV risk among RA patients with overall low estimated ASCVD risk with active inflammation.

Anti‐CCP indicates anti‐cyclic citrullinated peptide; RA, rheumatoid arthritis; RF, rheumatoid factor; hs‐CRP, high‐sensitivity C‐reactive protein; CDAI, clinical disease activity index; hs-cTNT, high sensitivity troponin

Figure 1. Survival curves for MACE among subjects with and without detectable hs-cTnT at baseline, stratified by cohort: hsCRP high->low cohort and hsCRP low->high.
B. Weber: Bristol-Myers Squibb(BMS), 1, Horizon Therapeutics, 1, Kiniksa, 1, Novo Nordisk, 1; D. Weisenfeld: None; M. Jeffway: None; J. Coblyn: None; M. Weinblatt: Abbvie, 2, 5, Aclaris, 2, Amgen, 2, Aqtual, 5, Bristol Myers Squibb, 2, 5, Canfite, 11, Corevitas, 2, CorEvitas, 2, Eli Lilly, 2, Gilead, 2, 2, Glaxo Smith Kline, 2, Horizon, 2, Inmedix, 11, Janssen, 5, Johnson and Johnson, 2, Pfizer, 2, Prometheus Laboratories, 2, Rani, 2, Revolo, 2, Sanofi, 2, Sci Rhom, 2, Scipher, 2, 11, Set Point, 2, UCB, 2; N. Shadick: Abbvie, 5, AQtual, 5, Bristol-Myers Squibb(BMS), 5, Janssen, 5; M. DiCarli: Amgen, 2, Gilead, 5, Medtrace, 2, sanofi, 2; K. Liao: UCB, 2.



