Poster Session A
Vasculitis
Session: (0691–0721) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
0697: Alterations in Composition and Function of Gut Microbiota in Patients with Large-Vessel Vasculitis
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- AM
Adam Mayer, MD
University of Pennsylvania/Children's Hospital of Philadelphia
Philadelphia, PA, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Adam Mayer1, Weiming Hu2, Kyle Bittinger2, Rui Liang3, Naomi Amudala3, Shubhasree Banerjee3, Peter Merkel3 and Rennie Rhee3, 1University of Pennsylvania/Children's Hospital of Philadelphia, Philadelphia, PA, 2Children's Hospital of Philadelphia, Philadelphia, PA, 3University of Pennsylvania, Philadelphia, PA
Background/Purpose: The gut microbiome has a critical role in immune homeostasis and there is growing interest in understanding the relationship between the microbiome and autoimmunity. There are epidemiologic and genetic associations between large-vessel vasculitis (LVV) and inflammatory bowel disease, an autoimmune disease where intestinal dysbiosis is common. This study evaluated the relationship between composition and function of gut microbiota and disease activity in patients with LVV.
Methods: In this longitudinal, prospective cohort study, stool samples and clinical data were collected from patients with giant cell arteritis (GCA) and Takayasu arteritis (TAK) every 6 months at a single center. Diagnosis and disease activity of LVV were determined by the investigator and informed by the 1990 ACR classification criteria and BVAS, respectively. Healthy control patients without autoimmune disease were also recruited and supplemented by existing healthy control stool microbiome data from a separate convenience cohort. The fecal microbiome was profiled using shotgun metagenomic sequencing. Comparisons to healthy controls were conducted using samples from the first visit at which: 1) LVV was inactive, and 2) LVV was active. The relative abundance of microbial taxa in the stool was estimated using Kraken2, and the relative abundance of microbial gene orthologs was determined by alignment to the Kyoto Encyclopedia of Genes and Genomes (KEGG). Community-level differences in taxonomic (beta diversity) and gene function composition in the microbiome were assessed using Bray-Curtis distances between samples in principle coordinate analysis (PCoA) plots. The relative abundance of the 10 most common functional pathways, determined by the abundance of constituent genes, was compared using heatmaps and linear models. P-values were adjusted to control for a false discovery rate of 5% when multiple comparisons were made.
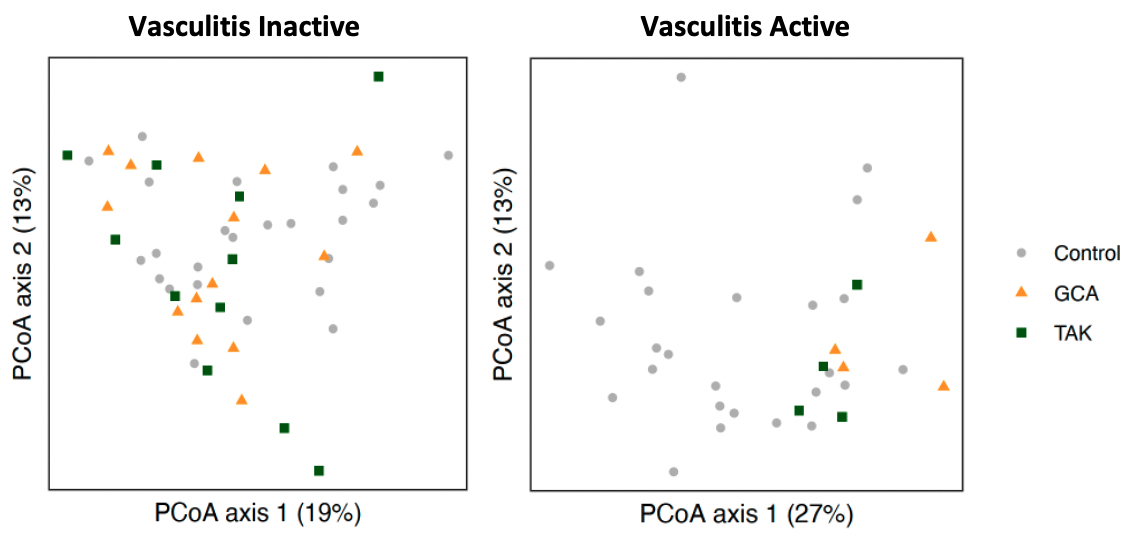
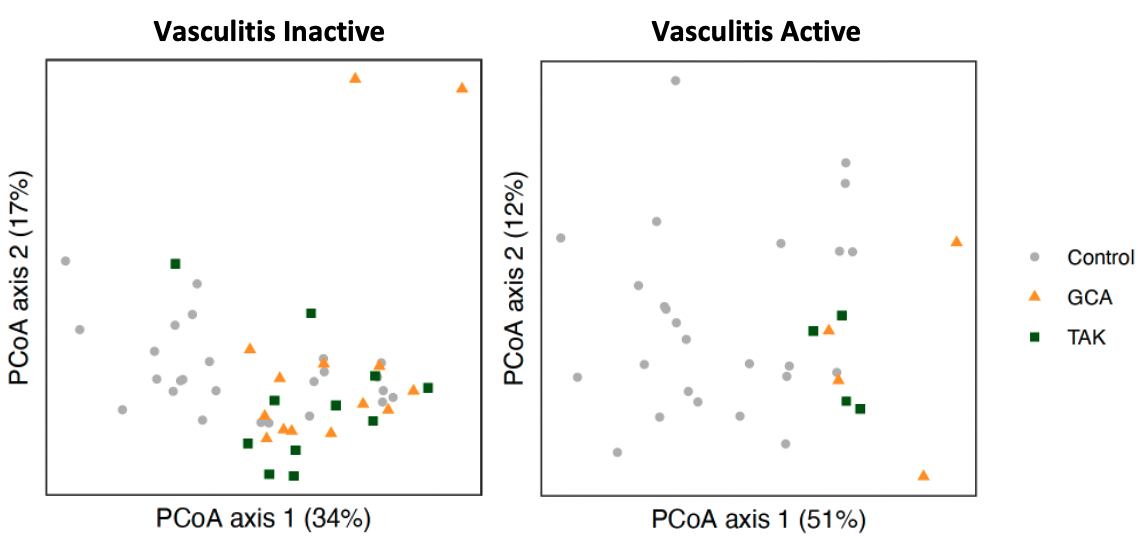
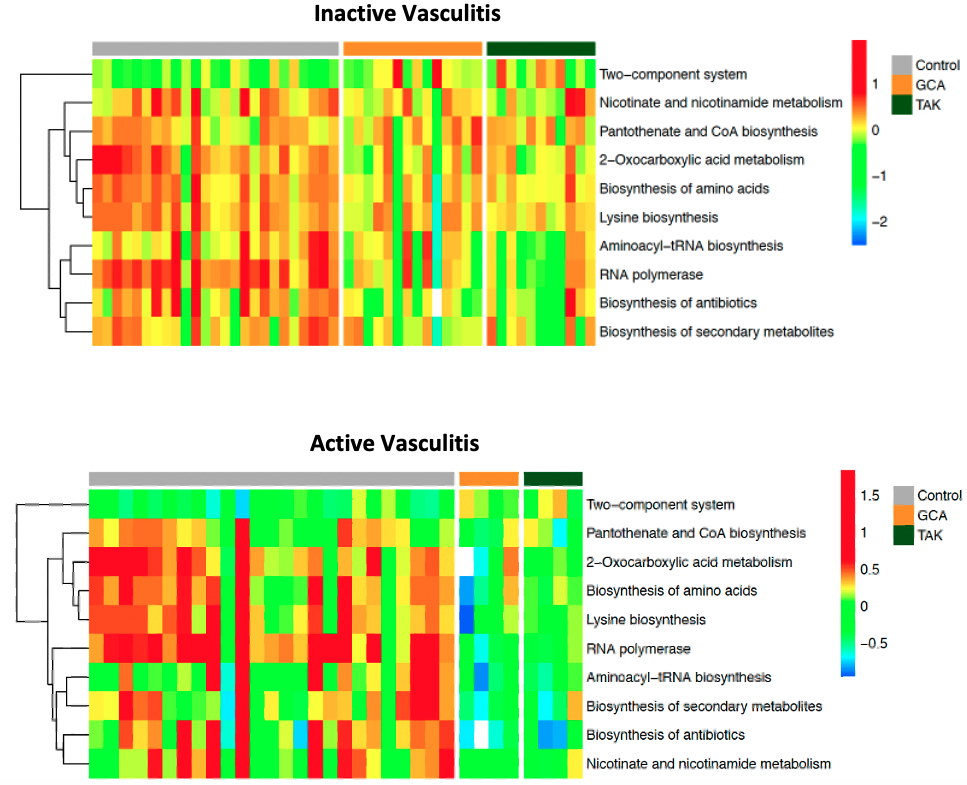
Results: 27 patients with LVV, which included 16 with GCA and 11 with TAK, and 23 healthy controls were included in the study with a total of 76 stool samples obtained. GCA patients were 75% female with mean age 70.4 years (SD 6.5) while TAK patients were all female with mean age 37.2 years (SD 13.8). Eight patients with LVV experienced a flare with the most common symptoms in GCA being constitutional symptoms (75%) and headache (50%) and in TAK chest pain/dyspnea (50%) and lower limb claudication (25%). Composition of fecal microbiota, measured by beta diversity, was significantly different in active LVV samples but not inactive LVV when compared to controls (Figure 1). In our analysis of microbial gene ortholog abundance, functional genes of bacteria were significantly different in both active and inactive LVV patients compared to controls (Figure 2). Several microbial gene pathways were identified to distinguish patients with active LVV from controls, but few emerged in our comparison of inactive LVV and controls (Figure 3).
Conclusion: Patients with LVV, particularly those with active disease, have altered composition, functional genes and pathways of gut microbiota compared to healthy controls. The possibility of shared mechanisms driving the altered microbiome in GCA and TAK warrants further investigation.
A. Mayer: None; W. Hu: None; K. Bittinger: None; R. Liang: None; N. Amudala: None; S. Banerjee: None; P. Merkel: AbbVie/Abbott, 5, Amgen, 2, 5, ArGenx, 2, AstraZeneca, 2, 5, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, Cabaletta, 2, CSL Behring, 2, Eicos, 5, Electra, 5, Genentech, 5, GlaxoSmithKlein(GSK), 2, 5, HiBio, 2, InflaRx, 2, 5, Janssen, 2, Jubilant, 2, Kyverna, 2, 11, MiroBio, 2, Neutrolis, 5, Novartis, 2, NS Pharma, 2, Q32, 2, Regeneron, 2, Sanofi, 2, Sparrow, 2, Takeda, 2, 5, UpToDate, 9, Visterra, 2; R. Rhee: None.
Background/Purpose: The gut microbiome has a critical role in immune homeostasis and there is growing interest in understanding the relationship between the microbiome and autoimmunity. There are epidemiologic and genetic associations between large-vessel vasculitis (LVV) and inflammatory bowel disease, an autoimmune disease where intestinal dysbiosis is common. This study evaluated the relationship between composition and function of gut microbiota and disease activity in patients with LVV.
Methods: In this longitudinal, prospective cohort study, stool samples and clinical data were collected from patients with giant cell arteritis (GCA) and Takayasu arteritis (TAK) every 6 months at a single center. Diagnosis and disease activity of LVV were determined by the investigator and informed by the 1990 ACR classification criteria and BVAS, respectively. Healthy control patients without autoimmune disease were also recruited and supplemented by existing healthy control stool microbiome data from a separate convenience cohort. The fecal microbiome was profiled using shotgun metagenomic sequencing. Comparisons to healthy controls were conducted using samples from the first visit at which: 1) LVV was inactive, and 2) LVV was active. The relative abundance of microbial taxa in the stool was estimated using Kraken2, and the relative abundance of microbial gene orthologs was determined by alignment to the Kyoto Encyclopedia of Genes and Genomes (KEGG). Community-level differences in taxonomic (beta diversity) and gene function composition in the microbiome were assessed using Bray-Curtis distances between samples in principle coordinate analysis (PCoA) plots. The relative abundance of the 10 most common functional pathways, determined by the abundance of constituent genes, was compared using heatmaps and linear models. P-values were adjusted to control for a false discovery rate of 5% when multiple comparisons were made.
Results: 27 patients with LVV, which included 16 with GCA and 11 with TAK, and 23 healthy controls were included in the study with a total of 76 stool samples obtained. GCA patients were 75% female with mean age 70.4 years (SD 6.5) while TAK patients were all female with mean age 37.2 years (SD 13.8). Eight patients with LVV experienced a flare with the most common symptoms in GCA being constitutional symptoms (75%) and headache (50%) and in TAK chest pain/dyspnea (50%) and lower limb claudication (25%). Composition of fecal microbiota, measured by beta diversity, was significantly different in active LVV samples but not inactive LVV when compared to controls (Figure 1). In our analysis of microbial gene ortholog abundance, functional genes of bacteria were significantly different in both active and inactive LVV patients compared to controls (Figure 2). Several microbial gene pathways were identified to distinguish patients with active LVV from controls, but few emerged in our comparison of inactive LVV and controls (Figure 3).
Conclusion: Patients with LVV, particularly those with active disease, have altered composition, functional genes and pathways of gut microbiota compared to healthy controls. The possibility of shared mechanisms driving the altered microbiome in GCA and TAK warrants further investigation.

Figure 1. Bacterial composition in large-vessel vasculitis (inactive and active) vs controls. Principal coordinate analysis (PCoA) plots compare beta diversity between controls vs inactive vasculitis (left panel) and active vasculitis (right panel) using Bray-Curtis distances. Beta diversity did not significantly differ when comparing inactive LVV to controls (p=0.21) but did between active vasculitis and controls (p<0.01).

Figure 2. Bacterial functional analysis in large-vessel vasculitis (inactive and active) vs controls. PCoA plots compare microbial metagenomic functional composition in inactive vasculitis (left panel) and active vasculitis (right panel) compared to controls using Bray-Curtis distances. These distributions significantly differed between controls and vasculitis subgroups both during inactive disease (p<0.01) and active disease (p<0.01).

Figure 3. Functional pathways of gut microbiota in large-vessel vasculitis vs controls. Heatmaps representing relative abundance of functional pathways between control patients, patients with giant cell arteritis, and patients with Takayasu arteritis. While few pathways significantly differed between groups during inactive vasculitis (top), more significant differences were observed from samples during active vasculitis (bottom).
A. Mayer: None; W. Hu: None; K. Bittinger: None; R. Liang: None; N. Amudala: None; S. Banerjee: None; P. Merkel: AbbVie/Abbott, 5, Amgen, 2, 5, ArGenx, 2, AstraZeneca, 2, 5, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, Cabaletta, 2, CSL Behring, 2, Eicos, 5, Electra, 5, Genentech, 5, GlaxoSmithKlein(GSK), 2, 5, HiBio, 2, InflaRx, 2, 5, Janssen, 2, Jubilant, 2, Kyverna, 2, 11, MiroBio, 2, Neutrolis, 5, Novartis, 2, NS Pharma, 2, Q32, 2, Regeneron, 2, Sanofi, 2, Sparrow, 2, Takeda, 2, 5, UpToDate, 9, Visterra, 2; R. Rhee: None.



