Abstract Session
Vasculitis
Session: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders II: Clinical (2557–2562)
2557: Clinicopathologic Features of Patients with Giant Cell Arteritis and Thoracic Aorta Repair: A Single Center Experience over Two Decades
Tuesday, November 14, 2023
4:00 PM - 4:10 PM PT
Location: Room 6F/6C
- MK
Presenting Author(s)
Mahmut Kaymakci1, Nicholas Boire1, Melanie Bois1, Mohanad Elfishawi1, Hannah Langenfeld2, Andrew Hanson2, Cynthia Crowson1, Matthew Koster1, Cornelia M. Weyand3 and Kenneth Warrington1, 1Mayo Clinic, Rochester, MN, 2Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN, 3Mayo Clinic School of Medicine and Stanford University, Rochester, MN
Background/Purpose: Patients with giant cell arteritis (GCA) are at increased risk of thoracic aortic aneurysm and dissection. This late complication of the disease is presumed to reflect damage from prior aortitis, however whether ongoing aortic inflammation contributes to aneurysm formation is largely unknown. The aim of this project was to investigate the clinicopathologic findings of patients with GCA who underwent thoracic aorta repair.
Methods: Patients who had thoracic aorta surgery at our institution between January 1, 2000, and December 31, 2021, were identified and screened for a prior diagnosis of GCA. The available data was manually abstracted from the medical records of all study subjects, and two cardiovascular pathologists reviewed available aorta tissue obtained during surgery. Grade 1 inflammation of the aorta was defined as inflammation limited to the peri-vasa vasorum or focal involvement, grade 2 was defined as inflammation beyond the peri-vasa vasorum or multifocal involvement but less than 50% of the medial thickness, grade 3 was defined as diffuse inflammation with involvement of equal or more than 50% of the medial thickness. Survival rates were estimated using Kaplan-Meier methods. Overall observed survival was compared with lifetable rates from the US population. The standardized mortality ratio was estimated as the ratio of the observed and expected number of deaths.
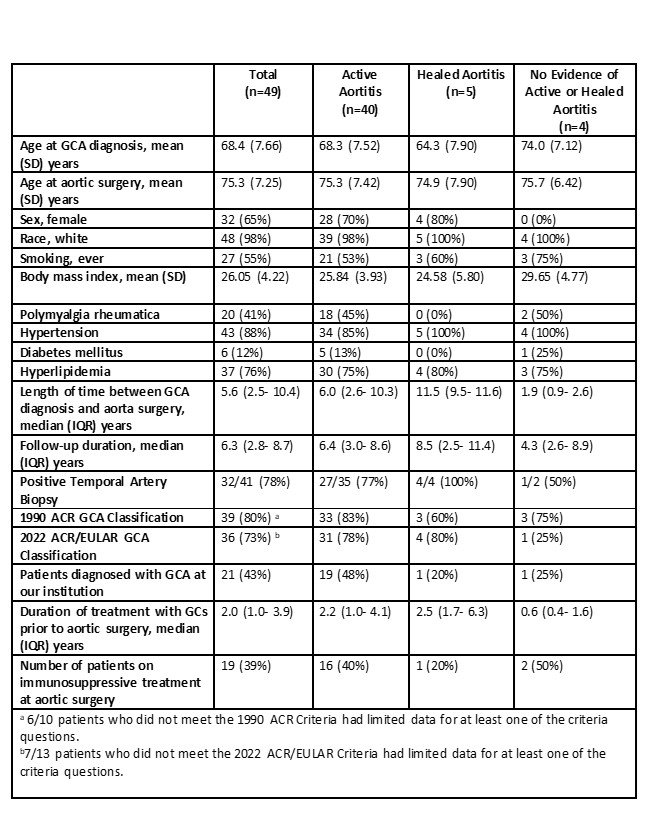
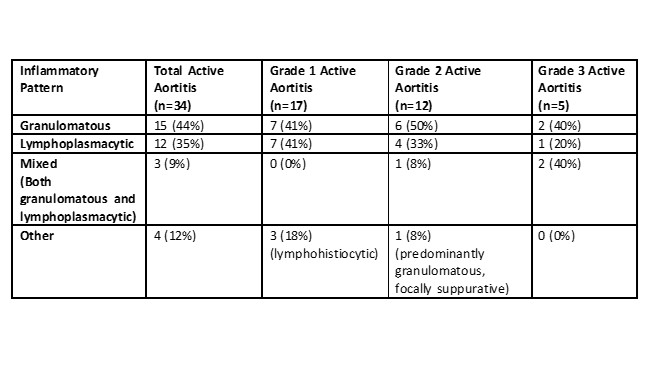
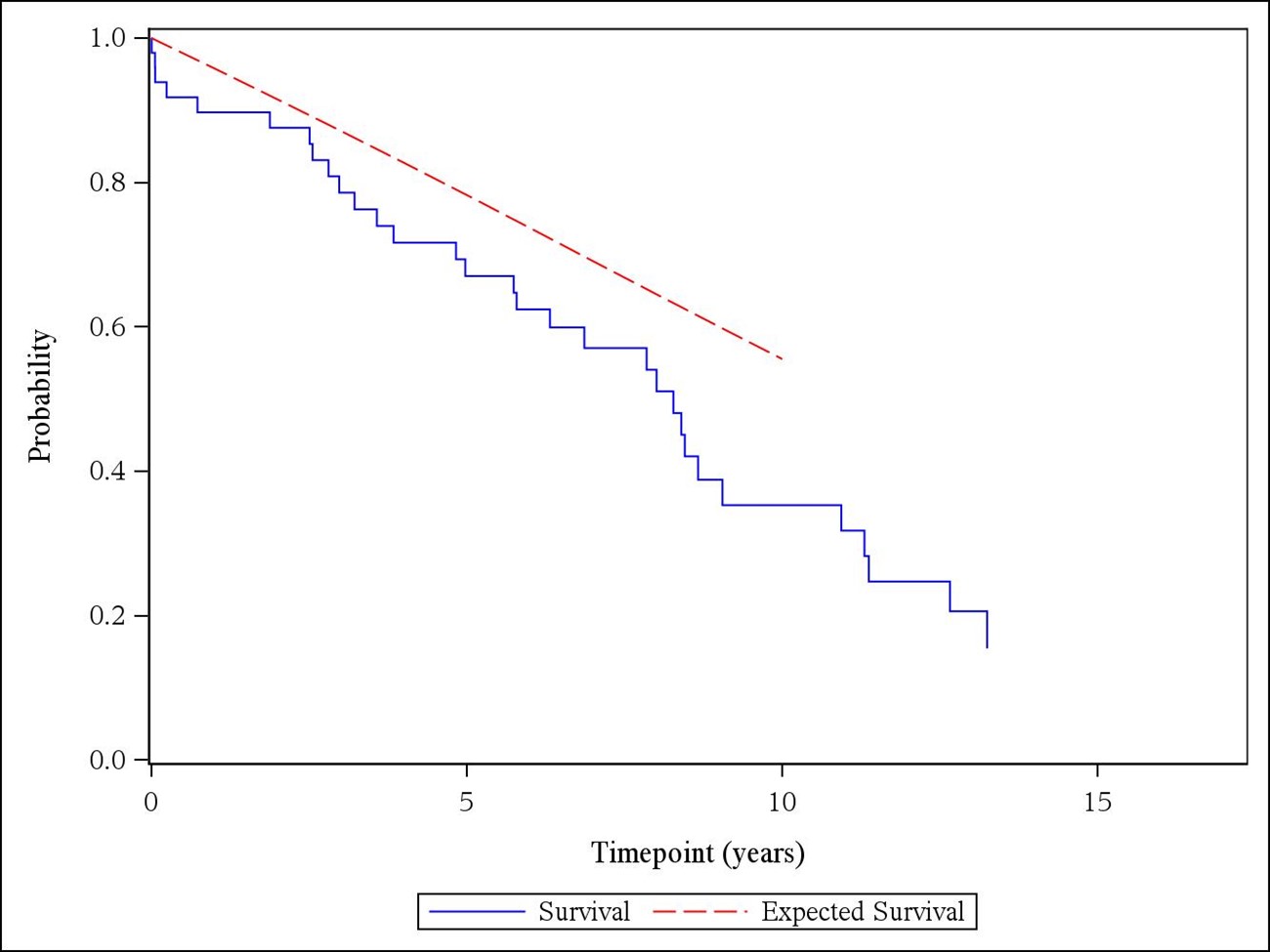
Results: Of the 4621 patients who underwent thoracic aorta surgery during the study period, 49 had a previous diagnosis of GCA (Table 1). Thirty-two (65%) were female, and 43 (88%) patients had either positive temporal artery biopsy or met the 1990 ACR or 2022 ACR/EULAR classification criteria for GCA. Forty-one patients had cranial symptoms at GCA diagnosis. All patients were considered in clinical remission at the time of aortic surgery. Ten (20%) patients had thoracic aortic dissection. Inflammatory markers (median [IQR]) were lower at the time of aortic surgery compared to initial diagnosis (erythrocyte sedimentation rate (mm/hr): 91 [54.0- 109.5] vs. 10 [5.0- 20.0]; C-reactive protein (mg/L): 76.5 [28.0- 139.0] vs. 3.1 [3.0- 8.0]). Histopathologic evaluation of the aortic tissue revealed active aortitis in most patients with GCA (40/49, 82%) after a median (IQR) of 6.0 (2.6- 10.3) years from GCA diagnosis. Healed aortitis was detected in 5 (10%) patients, and in 4 (8%) patients there was no evidence of active or healed aortitis. Nineteen (39%) patients were on immunosuppressive treatment at the time of aortic surgery. The detailed histopathologic re-evaluation of 43 out of 49 aortic samples revealed grade 1 aortitis in 17 (40%) patients, grade 2 in 12 (28%), and grade 3 in 5 (12%) (Table 2). The overall mortality compared to age and sex-matched general population was significantly increased with a standardized mortality ratio of 1.55 (95% CI, 1.05- 2.19) (Figure 1).
Conclusion: Histopathologic evaluation of the thoracic aorta obtained during surgery revealed active aortitis in most patients with GCA despite being considered in clinical remission several years after the initial diagnosis. Chronic, smoldering aortic inflammation likely contributes to the development of aortic aneurysm and dissection in GCA.
M. Kaymakci: None; N. Boire: None; M. Bois: None; M. Elfishawi: None; H. Langenfeld: None; A. Hanson: None; C. Crowson: None; M. Koster: None; C. Weyand: None; K. Warrington: Bristol-Myers Squibb(BMS), 5, Chemocentryx, 1, 6, Eli Lilly, 5, kiniksa, 5.
Background/Purpose: Patients with giant cell arteritis (GCA) are at increased risk of thoracic aortic aneurysm and dissection. This late complication of the disease is presumed to reflect damage from prior aortitis, however whether ongoing aortic inflammation contributes to aneurysm formation is largely unknown. The aim of this project was to investigate the clinicopathologic findings of patients with GCA who underwent thoracic aorta repair.
Methods: Patients who had thoracic aorta surgery at our institution between January 1, 2000, and December 31, 2021, were identified and screened for a prior diagnosis of GCA. The available data was manually abstracted from the medical records of all study subjects, and two cardiovascular pathologists reviewed available aorta tissue obtained during surgery. Grade 1 inflammation of the aorta was defined as inflammation limited to the peri-vasa vasorum or focal involvement, grade 2 was defined as inflammation beyond the peri-vasa vasorum or multifocal involvement but less than 50% of the medial thickness, grade 3 was defined as diffuse inflammation with involvement of equal or more than 50% of the medial thickness. Survival rates were estimated using Kaplan-Meier methods. Overall observed survival was compared with lifetable rates from the US population. The standardized mortality ratio was estimated as the ratio of the observed and expected number of deaths.
Results: Of the 4621 patients who underwent thoracic aorta surgery during the study period, 49 had a previous diagnosis of GCA (Table 1). Thirty-two (65%) were female, and 43 (88%) patients had either positive temporal artery biopsy or met the 1990 ACR or 2022 ACR/EULAR classification criteria for GCA. Forty-one patients had cranial symptoms at GCA diagnosis. All patients were considered in clinical remission at the time of aortic surgery. Ten (20%) patients had thoracic aortic dissection. Inflammatory markers (median [IQR]) were lower at the time of aortic surgery compared to initial diagnosis (erythrocyte sedimentation rate (mm/hr): 91 [54.0- 109.5] vs. 10 [5.0- 20.0]; C-reactive protein (mg/L): 76.5 [28.0- 139.0] vs. 3.1 [3.0- 8.0]). Histopathologic evaluation of the aortic tissue revealed active aortitis in most patients with GCA (40/49, 82%) after a median (IQR) of 6.0 (2.6- 10.3) years from GCA diagnosis. Healed aortitis was detected in 5 (10%) patients, and in 4 (8%) patients there was no evidence of active or healed aortitis. Nineteen (39%) patients were on immunosuppressive treatment at the time of aortic surgery. The detailed histopathologic re-evaluation of 43 out of 49 aortic samples revealed grade 1 aortitis in 17 (40%) patients, grade 2 in 12 (28%), and grade 3 in 5 (12%) (Table 2). The overall mortality compared to age and sex-matched general population was significantly increased with a standardized mortality ratio of 1.55 (95% CI, 1.05- 2.19) (Figure 1).
Conclusion: Histopathologic evaluation of the thoracic aorta obtained during surgery revealed active aortitis in most patients with GCA despite being considered in clinical remission several years after the initial diagnosis. Chronic, smoldering aortic inflammation likely contributes to the development of aortic aneurysm and dissection in GCA.

Table 1. Characteristics of the patients with giant cell arteritis who had thoracic aorta surgery.

Table 2. Detailed histopathologic re-evaluation of 34 aorta specimens showing active aortitis.

Figure 1. Overall survival of the patients with giant cell arteritis compared to expected rates from United States total lifetables (observed: solid blue line; expected: dashed red line).
M. Kaymakci: None; N. Boire: None; M. Bois: None; M. Elfishawi: None; H. Langenfeld: None; A. Hanson: None; C. Crowson: None; M. Koster: None; C. Weyand: None; K. Warrington: Bristol-Myers Squibb(BMS), 5, Chemocentryx, 1, 6, Eli Lilly, 5, kiniksa, 5.



