Poster Session A
Vasculitis
Session: (0673–0690) Vasculitis – ANCA-Associated Poster I: Treatment Outcomes
0678: Benralizumab in Eosinophilic Granulomatosis with Polyangiitis
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- AC
Adrien Cottu, MD, -None-
Sorbonne Université
Boulogne-Billancourt, FranceDisclosure information not submitted.
Abstract Poster Presenter(s)
Adrien Cottu1, Matthieu Groh2, Charlène Desaintjean3, Sylvain Marchand-Adam4, Loic Guillevin5, Xavier Puéchal6, Estibaliz Lazaro7, Maxime Samson8, Camille Taillé9, Cécile-Audrey Durel10, Elisabeth Diot11, Sarah Nicolas11, Laurent Guilleminault12, Mikael Ebbo13, Pascal Cathébras14, Clairelyne Dupin9, Halil Yildiz15, Nabil Belfeki16, Gregory Pugnet17, Pierre Chauvin18, Stephane Jouneau19, François Lifermann20, Jean-Philippe Martellosio21, Vincent Cottin22 and Benjamin Terrier23, 1Department of Internal Medicine, Hôpital Cochin, Assistance Publique-Hôpitaux de Paris (AP-HP), Paris, France, 2National Referral Center for Hypereosinophilic Syndrome (CEREO), Hôpital Foch, Suresnes, France, 3Department of Respiratory Diseases, Hôpital Louis Pradel, Lyon, France, 4CHRU Tours, service de pneumologie et d'explorations fonctionnelles respiratoires, Tours, France, 5University Paris Descartes, Paris, France, 6National Referral Center for Rare Systemic Autoimmune Diseases, Paris, France, 7Bordeaux Hospital University, Pessac, France, 8Department of Internal Medicine and Clinical Immunology, Dijon University Hospital, Dijon, France, 9AP-HP, Bichat Hospital, Reference Center for Rare Pulmonary Diseases and University of Paris Cité, Inserm 1152, Paris, France, 10CHU Lyon, Lyon, France, 11Service de médecine interne et immunologie clinique, CHU Tours, Tours, France, 12Department of respiratory and allergic diseases, Toulouse University Hospital Center, Toulouse, France, 13Aix Marseille Univ, APHM, hôpital de la Timone, Internal Medicine Department, Marseille, France, 14Department of Internal medicine, Hôpital Nord, CHU St Etienne, Saint-Etienne, France, 15Saint Luc Hospital, Bruxelles, Belgium, 16Service de Médecine Interne et Immunologie Clinique. Groupe Hospitalier Sud Île de France, Melun, France, 17CHU Toulouse Rangueil Service de Medecine Interne et Immunologie Clinique, Toulouse, France, 18Department of Respiratory Diseases, Rennes University Hospital, Rennes, France, 19Amicus, Boca Raton, FL, 20Department of Internal Medicine, Centre Hospitalier de Dax, Dax, France, 21Service de médecine interne, maladies infectieuses et tropicales, CHU de Poitiers, Poitiers, France, 22Coordinating Reference Center for Rare Pulmonary Diseases, Louis Pradel Hospital, University of Lyon, INRAE, Lyon, France, 23Department of Internal Medicine, Hôpital Cochin, AP-HP, Paris, France
Background/Purpose: Gucocorticoid (GC)-dependant asthma and ENT exacerbations may persist in more than 80% of eosinophilic granulomatosis with polyangiitis (EGPA). The MIRRA trial demonstrated the efficacy of mepolizumab, a monoclonal antibody targeting interleukin-5 (IL-5), in treating these manifestations. However, roughly 70% of patients still required more than 4 mg per day of prednisone at the end of follow-up or relapsed. Benralizumab, a monoclonal-antibody targeting IL-5 receptor, causes profound eosinophil depletion in plasma, sputum, and tissues. However, its efficacy in GC-dependent manifestations remains to be determined in EGPA. We aimed to describe the use and efficacy of benralizumab in patients with refractory manifestations of EGPA.
Methods: We conducted a multicentre, retrospective study of patients with EGPA according to ACR/EULAR 2022 criteria and treated with benralizumab between February 2019 and February 2023. Complete response was defined as no disease activity (BVAS=0) and a prednisone dose ≤4 mg/day. Partial response was defined as no disease activity and a prednisone dose ≥4 mg/day. Total response was defined by either complete or partial response. Comparisons between patients with and without prior mepolizumab therapy were made using the Fisher exact test and the Mann Whitney test. Comparisons of responses or time to vasculitis flares were made with log-rank test.
Results: Sixty-eight patients were included. The median age was 50 (IQR 39-63) years. ANCA were positive in 20 (29%) patients at EGPA diagnosis. Thirty-one patients (46%) had previously received mepolizumab, at a dose of 100 mg/month in 18/26 (69%) and 300 mg/month in 8/26 (31%). Primary or secondary failure of mepolizumab accounted for 16 patients (52%) and 15 patients (48%). The use of benralizumab was justified by uncontrolled asthma in 54 (81%), uncontrolled ENT manifestations in 27 (40%) and persistent glucocorticoid use in 48 (74%) patients. The asthma regimen (30 mg/month 3 times then every 2 months) was used in 63/66 patients (95%). Sixteen patients (24%) were concomitantly treated with another immunosuppressant.
Median follow-up after initiation of benralizumab was 23 months (IQR 9-34). Thirty-three patients (49%) had a complete response, 24 (36%) had a partial response and 10 (15%) failed to respond (response assessed in 67 patients). Of 57 patients with at least a partial initial response, 10 (18%) experienced secondary failure. GCs were discontinued in 23 patients (38%). Prior mepolizumab use was associated with more primary failure (p=0.034) and less GC discontinuation (p=0.001).
Vasculitis flares occurred in 7 patients (11%) and were associated with histological evidence of vasculitis and/or ANCA positivity at benralizumab initiation (p=0.010). Six infections requiring specific treatments were reported during follow-up.
Conclusion: Benralizumab appears to be an effective treatment for refractory asthma or ENT manifestations in EGPA and allows glucocorticoid withdrawal. However, its efficacy was lower after prior mepolizumab failure.
A. Cottu: None; M. Groh: AstraZeneca, 6, GSK, 6, Sanofi, 6; C. Desaintjean: None; S. Marchand-Adam: None; L. Guillevin: None; X. Puéchal: None; E. Lazaro: None; M. Samson: ARGENX, 2, Boehringer-Ingelheim, 2, CHUGAI, 2, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2, NOVARTIS, 2, 5; C. Taillé: AstraZeneca, 6, Chiesi, 5, 6, GSK, 5, 6, Novartis, 5, 6, Sanofi, 6; C. Durel: None; E. Diot: None; S. Nicolas: None; L. Guilleminault: None; M. Ebbo: None; P. Cathébras: None; C. Dupin: AstraZeneca, 6, GSK, 6; H. Yildiz: GSK, 6; N. Belfeki: None; G. Pugnet: None; P. Chauvin: None; S. Jouneau: None; F. Lifermann: None; J. Martellosio: None; V. Cottin: Boehringer Ingelheim, 2, 5, 6, 12, Support for attending meetings, Celgene/BMS, 1, 2, CSL Behring, 2, Ferrer, 2, 6, 12, Support for attending meetings, FibroGen, 1, Galapagos, 1, 2, Galecto, 1, GlaxoSmithKline, 2, Pliant, 2, Pure Tech, 2, Redx, 2, Roche, 1, 2, 6, 12, Support for attending meetings, Sanofi, 2, Shionogi, 2; B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2.
Background/Purpose: Gucocorticoid (GC)-dependant asthma and ENT exacerbations may persist in more than 80% of eosinophilic granulomatosis with polyangiitis (EGPA). The MIRRA trial demonstrated the efficacy of mepolizumab, a monoclonal antibody targeting interleukin-5 (IL-5), in treating these manifestations. However, roughly 70% of patients still required more than 4 mg per day of prednisone at the end of follow-up or relapsed. Benralizumab, a monoclonal-antibody targeting IL-5 receptor, causes profound eosinophil depletion in plasma, sputum, and tissues. However, its efficacy in GC-dependent manifestations remains to be determined in EGPA. We aimed to describe the use and efficacy of benralizumab in patients with refractory manifestations of EGPA.
Methods: We conducted a multicentre, retrospective study of patients with EGPA according to ACR/EULAR 2022 criteria and treated with benralizumab between February 2019 and February 2023. Complete response was defined as no disease activity (BVAS=0) and a prednisone dose ≤4 mg/day. Partial response was defined as no disease activity and a prednisone dose ≥4 mg/day. Total response was defined by either complete or partial response. Comparisons between patients with and without prior mepolizumab therapy were made using the Fisher exact test and the Mann Whitney test. Comparisons of responses or time to vasculitis flares were made with log-rank test.
Results: Sixty-eight patients were included. The median age was 50 (IQR 39-63) years. ANCA were positive in 20 (29%) patients at EGPA diagnosis. Thirty-one patients (46%) had previously received mepolizumab, at a dose of 100 mg/month in 18/26 (69%) and 300 mg/month in 8/26 (31%). Primary or secondary failure of mepolizumab accounted for 16 patients (52%) and 15 patients (48%). The use of benralizumab was justified by uncontrolled asthma in 54 (81%), uncontrolled ENT manifestations in 27 (40%) and persistent glucocorticoid use in 48 (74%) patients. The asthma regimen (30 mg/month 3 times then every 2 months) was used in 63/66 patients (95%). Sixteen patients (24%) were concomitantly treated with another immunosuppressant.
Median follow-up after initiation of benralizumab was 23 months (IQR 9-34). Thirty-three patients (49%) had a complete response, 24 (36%) had a partial response and 10 (15%) failed to respond (response assessed in 67 patients). Of 57 patients with at least a partial initial response, 10 (18%) experienced secondary failure. GCs were discontinued in 23 patients (38%). Prior mepolizumab use was associated with more primary failure (p=0.034) and less GC discontinuation (p=0.001).
Vasculitis flares occurred in 7 patients (11%) and were associated with histological evidence of vasculitis and/or ANCA positivity at benralizumab initiation (p=0.010). Six infections requiring specific treatments were reported during follow-up.
Conclusion: Benralizumab appears to be an effective treatment for refractory asthma or ENT manifestations in EGPA and allows glucocorticoid withdrawal. However, its efficacy was lower after prior mepolizumab failure.

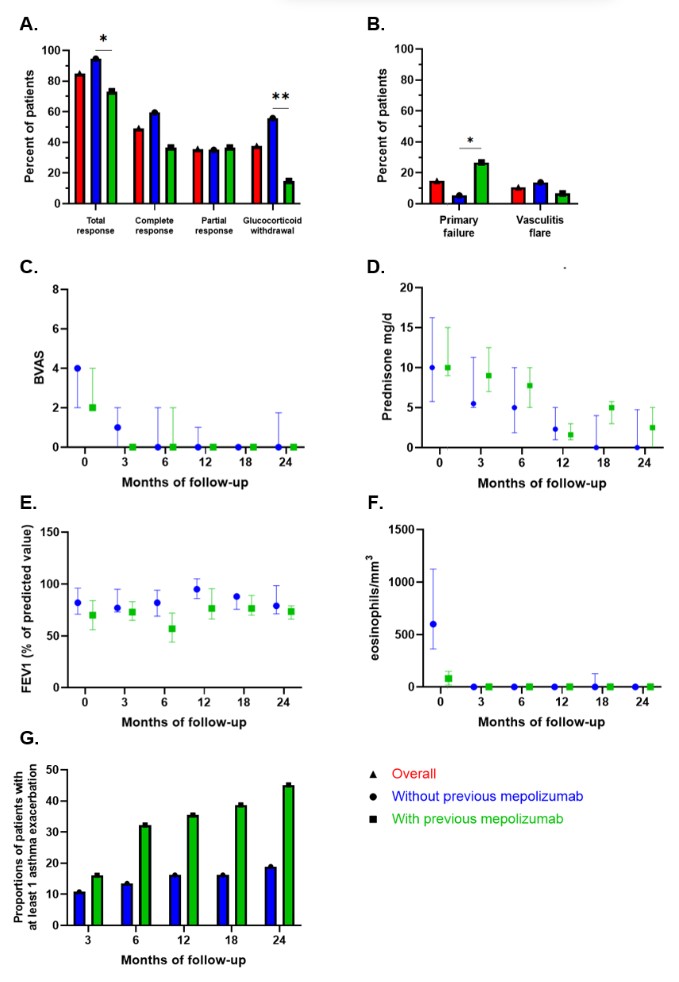
Response to benralizumab (A) and failure to benralizumab (B) among patients with eosinophilic granulomatosis with polyangiitis, according to previous treatment by mepolizumab. Variation in disease activity using the Birmingham Vasculitis Activity Score (BVAS) (C), daily dose of prednisone (D), variation in the forced expiratory volume in 1 second (FEV1) (E) and eosinophils count (F) among patients with eosinophilic granulomatosis with polyangiitis treated with benralizumab, according to previous treatment by mepolizumab. Accumulated proportions of patient with at least one asthma exacerbations during follow-up (G). Values in C, D, E, and F are the median and interquartile range.
IS = immunosuppressant; * = P < 0.05; ** = P < 0.01 (Fisher exact test).
IS = immunosuppressant; * = P < 0.05; ** = P < 0.01 (Fisher exact test).

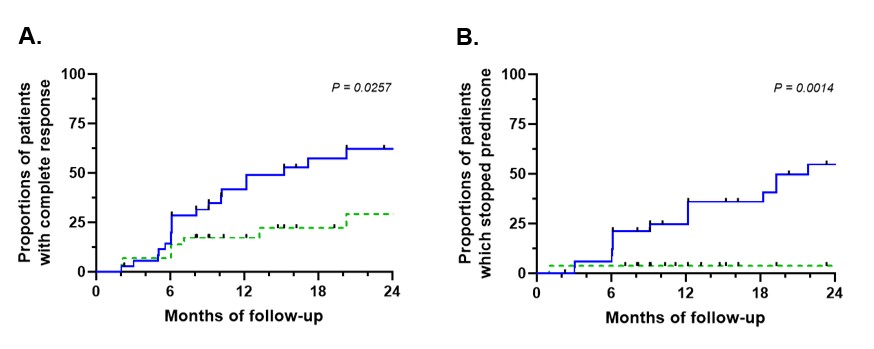
Kaplan-Meier survival curve of complete response (A) and withdrawal of glucocorticoids (B) among patients with eosinophilic granulomatosis with polyangiitis treated with benralizumab, according to previous treatment by mepolizumab. Blue full line = without previous mepolizumab; green dotted line = with previous mepolizumab. (Logrank test)
A. Cottu: None; M. Groh: AstraZeneca, 6, GSK, 6, Sanofi, 6; C. Desaintjean: None; S. Marchand-Adam: None; L. Guillevin: None; X. Puéchal: None; E. Lazaro: None; M. Samson: ARGENX, 2, Boehringer-Ingelheim, 2, CHUGAI, 2, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2, NOVARTIS, 2, 5; C. Taillé: AstraZeneca, 6, Chiesi, 5, 6, GSK, 5, 6, Novartis, 5, 6, Sanofi, 6; C. Durel: None; E. Diot: None; S. Nicolas: None; L. Guilleminault: None; M. Ebbo: None; P. Cathébras: None; C. Dupin: AstraZeneca, 6, GSK, 6; H. Yildiz: GSK, 6; N. Belfeki: None; G. Pugnet: None; P. Chauvin: None; S. Jouneau: None; F. Lifermann: None; J. Martellosio: None; V. Cottin: Boehringer Ingelheim, 2, 5, 6, 12, Support for attending meetings, Celgene/BMS, 1, 2, CSL Behring, 2, Ferrer, 2, 6, 12, Support for attending meetings, FibroGen, 1, Galapagos, 1, 2, Galecto, 1, GlaxoSmithKline, 2, Pliant, 2, Pure Tech, 2, Redx, 2, Roche, 1, 2, 6, 12, Support for attending meetings, Sanofi, 2, Shionogi, 2; B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2.



