Poster Session A
Crystal arthropathies
Session: (0229–0251) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
0234: Identifying Optimal Serum Urate Levels to Reduce Gout Flares in Patients Taking Urate Lowering Therapy: A Post-hoc Cohort Analysis of CARES with Consideration of Drop-out
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
.png)
Sara Tedeschi, MD, MPH
Brigham and Women's Hospital
Boston, MA, United StatesDisclosure(s): Novartis: Consultant (Terminated, March 1, 2023)
Abstract Poster Presenter(s)
Sara Tedeschi1, Keigo Hayashi1, Yuqing Zhang2, Hyon K. Choi3 and Daniel Solomon1, 1Brigham and Women's Hospital, Boston, MA, 2Division of Rheumatology, Allergy, and Immunology, Department of Medicine, Massachusetts General Hospital, Boston, MA, 3Massachusetts General Hospital and Harvard Medical School, Lexington, MA
Background/Purpose: ACR gout treatment guidelines recommend a target serum urate (SU) of < 6 mg/dL and anti-inflammatory flare prophylaxis for at least 3-6 months after initiating urate-lowering therapy (ULT). However, optimal targets for SU are not well defined. We investigated gout flare rates based on repeated measurements of SU levels in a randomized controlled trial of ULT, accounting for loss to follow up.
Methods: We performed a secondary analysis using data from the Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout (CARES) trial. CARES participants were randomized to febuxostat or allopurinol, titrated for a target SU < 6 mg/dL; 45% did not complete all study visits. Participants received gout flare prophylaxis for 6 months with colchicine 0.6 mg daily or naproxen 250 mg twice daily if colchicine was not tolerated. For this analysis, participants were followed from month 0 (randomization) to the earliest of death, last completed visit (drop out), or end of study. SU levels were assessed at months 0, 3, 6 and then every 6 months and categorized as ≤3.9, 4.0-5.9, 6.0-7.9, 8.0-9.9, and ≥10 mg/dL. The primary outcome was self-reported gout flare in each 3- or 6-month interval. More than 1 flare/interval was permitted if they were separated by ≥14 days. Baseline variables were used to derive inverse probability of censoring weights (IPCW) to account for censoring (drop-out or death). Poisson regression models included stabilized IPCW weights and estimated gout flare incidence rate ratios (IRR) by time-varying SU category, adjusting for change in SU from prior visit, flare prophylaxis, ULT, age, sex, race, body mass index, gout duration, and tophi. Models were performed for months 0-6, 6-12, and 12-72.
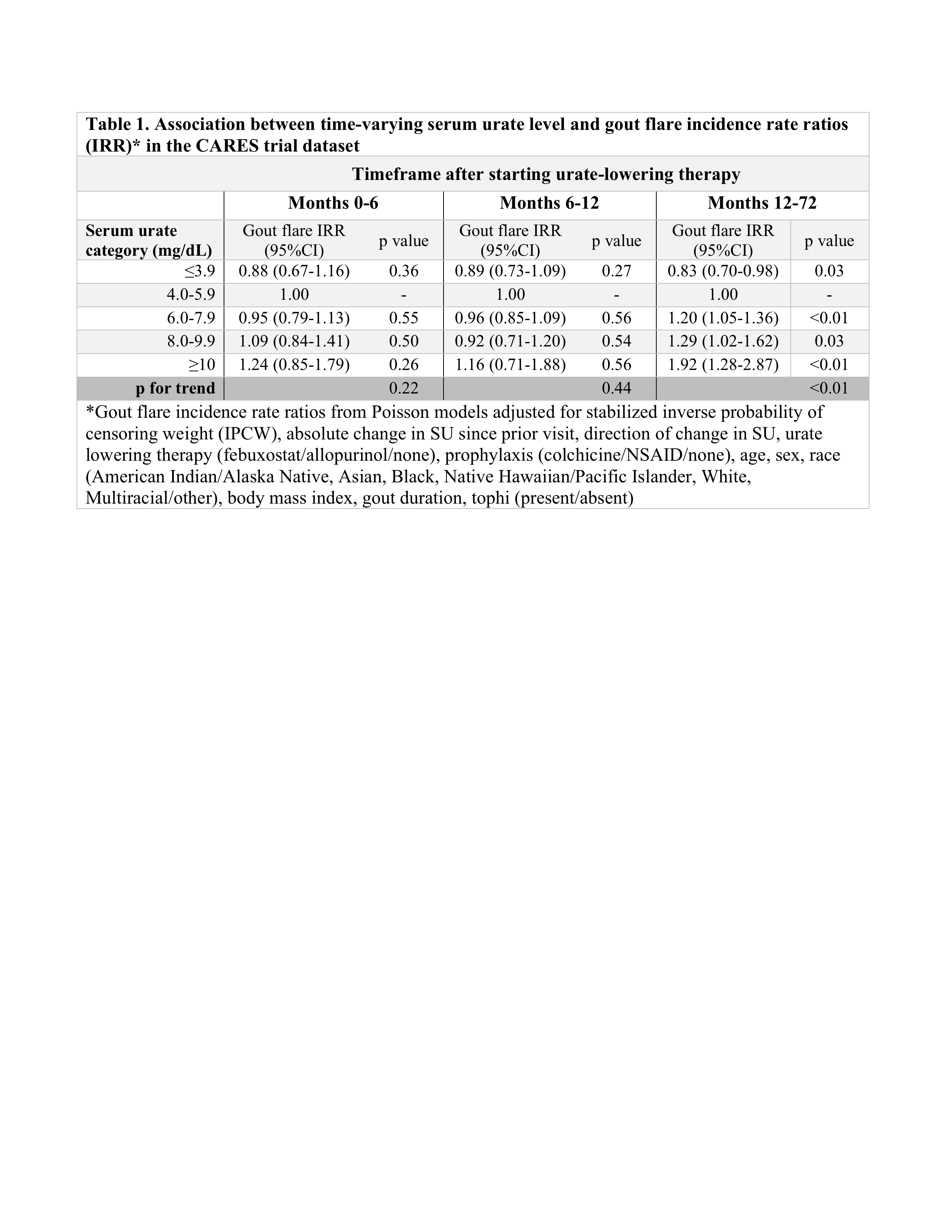
Results: Among 6183 participants in this analysis, median age was 65 (IQR 58-71) years and 84.0% were male. Median follow-up was 32 months. At month 0, median SU was 8.6 (IQR 7.6-9.7) mg/dL. Seventy-one percent achieved SU < 6 mg/dL by month 3, and this percentage increased slightly over time among retained participants (Figures 1A & 1B). Gout flare rates were highest during nearly all intervals when SU ≥10 mg/dL and lowest when SU ≤3.9 mg/dL (Figure 2). Peak gout flare rates for all SU categories were observed between months 0-3, coinciding with the initiation of ULT and greatest change in SU. A second spike in gout flares occurred in all SU groups between months 6-12, coinciding with discontinuation of prophylaxis (Figure 2). In the initial year of ULT, flare rates did not significantly differ between SU groups, but flare rates were consistently highest when SU ≥10 mg/dL. During months 12-72, in adjusted analyses with IPCW weights, a dose-response relationship was observed between SU category and flare rate. Compared with SU 4.0-5.9 mg/dL, significantly lower flare rates were observed when SU ≤3.9 mg/dL and significantly greater rates when SU ≥10 mg/dL (p for trend < 0.01) (Table 1).
Conclusion: Gout flare rates were persistently higher when SU ≥6 mg/dL compared to SU at target after the first year of ULT, after accounting for censoring. These data suggest a potential benefit of achieving very low SU levels (≤3.9 mg/dL) and consideration of a longer duration of prophylaxis to reduce gout flares.
.jpg)
.jpg)
S. Tedeschi: Novartis, 2; K. Hayashi: None; Y. Zhang: None; H. Choi: Ani, 2, Horizon, 2, 5, LG, 2, Protalix, 2, Shanton, 2; D. Solomon: CorEvitas, 5, Janssen, 5, moderna, 5, Novartis, 5, UpToDate, 9.
Background/Purpose: ACR gout treatment guidelines recommend a target serum urate (SU) of < 6 mg/dL and anti-inflammatory flare prophylaxis for at least 3-6 months after initiating urate-lowering therapy (ULT). However, optimal targets for SU are not well defined. We investigated gout flare rates based on repeated measurements of SU levels in a randomized controlled trial of ULT, accounting for loss to follow up.
Methods: We performed a secondary analysis using data from the Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout (CARES) trial. CARES participants were randomized to febuxostat or allopurinol, titrated for a target SU < 6 mg/dL; 45% did not complete all study visits. Participants received gout flare prophylaxis for 6 months with colchicine 0.6 mg daily or naproxen 250 mg twice daily if colchicine was not tolerated. For this analysis, participants were followed from month 0 (randomization) to the earliest of death, last completed visit (drop out), or end of study. SU levels were assessed at months 0, 3, 6 and then every 6 months and categorized as ≤3.9, 4.0-5.9, 6.0-7.9, 8.0-9.9, and ≥10 mg/dL. The primary outcome was self-reported gout flare in each 3- or 6-month interval. More than 1 flare/interval was permitted if they were separated by ≥14 days. Baseline variables were used to derive inverse probability of censoring weights (IPCW) to account for censoring (drop-out or death). Poisson regression models included stabilized IPCW weights and estimated gout flare incidence rate ratios (IRR) by time-varying SU category, adjusting for change in SU from prior visit, flare prophylaxis, ULT, age, sex, race, body mass index, gout duration, and tophi. Models were performed for months 0-6, 6-12, and 12-72.
Results: Among 6183 participants in this analysis, median age was 65 (IQR 58-71) years and 84.0% were male. Median follow-up was 32 months. At month 0, median SU was 8.6 (IQR 7.6-9.7) mg/dL. Seventy-one percent achieved SU < 6 mg/dL by month 3, and this percentage increased slightly over time among retained participants (Figures 1A & 1B). Gout flare rates were highest during nearly all intervals when SU ≥10 mg/dL and lowest when SU ≤3.9 mg/dL (Figure 2). Peak gout flare rates for all SU categories were observed between months 0-3, coinciding with the initiation of ULT and greatest change in SU. A second spike in gout flares occurred in all SU groups between months 6-12, coinciding with discontinuation of prophylaxis (Figure 2). In the initial year of ULT, flare rates did not significantly differ between SU groups, but flare rates were consistently highest when SU ≥10 mg/dL. During months 12-72, in adjusted analyses with IPCW weights, a dose-response relationship was observed between SU category and flare rate. Compared with SU 4.0-5.9 mg/dL, significantly lower flare rates were observed when SU ≤3.9 mg/dL and significantly greater rates when SU ≥10 mg/dL (p for trend < 0.01) (Table 1).
Conclusion: Gout flare rates were persistently higher when SU ≥6 mg/dL compared to SU at target after the first year of ULT, after accounting for censoring. These data suggest a potential benefit of achieving very low SU levels (≤3.9 mg/dL) and consideration of a longer duration of prophylaxis to reduce gout flares.

.jpg)
.jpg)
S. Tedeschi: Novartis, 2; K. Hayashi: None; Y. Zhang: None; H. Choi: Ani, 2, Horizon, 2, 5, LG, 2, Protalix, 2, Shanton, 2; D. Solomon: CorEvitas, 5, Janssen, 5, moderna, 5, Novartis, 5, UpToDate, 9.



