Poster Session A
Vasculitis
Session: (0691–0721) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
0696: Association of Large-vessel Vasculitis with Inflammatory Bowel Diseases: A European Case-control Study
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- FM
François Maillet, MD, MSc (he/him/his)
University of Paris
Asnières-sur-Seine, FranceDisclosure information not submitted.
Abstract Poster Presenter(s)
Francois Maillet1, Yann Nguyen2, Olivier Espitia3, Laurent Perard4, Carlo Salvarani5, Etienne Rivière6, Cécile-Audrey Durel7, Philippe GUILPAIN8, Luc Mouthon9, Anna Kernder10, Javier Loricera11, Pascal Cohen12, Isabelle Melki13, Claire De Moreuil14, Nicolas Limal15, Arsène Mekinian16, Nathalie Costedoat-Chalumeau17, Nathalie Morel12, Jonathan Boutemy18, Loic Raffray19, Jean-Sébastien Allain20, Valerie Devauchelle21, Isabelle Kone-Paut22, Marc Fabre23, Marie Durel24, Antoine Dossier25, Sébastien Abad26, Marcella Visentini27, Adrien Bigot28, Halil Yildiz29, Olivier Fain30, Maxime Samson31, Guillaume Gondran32, Vered Abitbol1 and Benjamin Terrier33, 1Cochin Hospital, Paris, France, 2Department of Internal Medicine, Hôpital Beaujon, AP-HP, Clichy, France., Montmorency, France, 3CHU de Nantes, Nantes, France, 4CH Saint Hoseph Saint Luc, Lyon, France, 5Azienda USL -IRCCS di Reggio Emilia and Università di Modena e Reggio Emilia, Reggio Emilia, Italy, 6CHU Bordeaux, Bordeaux, France, 7CHU Lyon, Lyon, France, 8University of Montpellier, Montpellier, France, 9Hopital Cochin - Paris University, Paris, France, 10HHU Düsseldorf, Düsseldorf, Germany, 11Hospital Universitario Marqués de Valdecilla, Santander, Spain, 12CHU Cochin, Paris, France, 13Hopital Robert Debré, Sérurier, France, 14CHU de Brest, Brest, France, 15AP-HP, Créteil, France, 16Department of Internal Medicine, Hôpital Saint-Antoine, AP-HP, Paris, France, 17Inserm DR Paris 5, Paris, France, 18CHU de Caen, Caen, France, 19CHU la Réunion, La Réunion, France, 20CHU Rennes, Rennes, France, 21UBO, Brest, France, 22Department of Pediatric Rheumatology, Reference Centre for Autoinflammatory Disorders CEREMAIA, Bicêtre Hospital, APHP, University of Paris Saclay, Paris, France, 23CH Bourgoin-Jallieu, Bourgoin-Jallieu, France, 24CH Metz, Metz, France, 25AP-HP, Paris, France, 26Avicenne Hospital, Bobigny, France, 27Rome Hospital, Rome, France, 28Tours Hospital, Tours, France, 29Saint Luc Hospital, Bruxelles, Belgium, 30Hopital SAINT ANTOINE APHP, Paris, France, 31Department of Internal Medicine and Clinical Immunology, Dijon University Hospital, Dijon, France, 32Limoges Hospital, Limoges, France, 33Department of Internal Medicine, Hôpital Cochin, AP-HP, Paris, France
Background/Purpose: The association of large vessel vasculitis (LVV), whether Takayasu arteritis (TA) or giant cell arteritis (GCA), with inflammatory bowel disease (IBD) is a rare and challenging condition. The prevalence of LVV in IBD cohorts is less than 1%, but the prevalence of IBD can be as high as 15.4% in TA cohorts, and population-based studies confirmed this association. We aimed to describe the characteristics and outcome of LVV-IBD patients, with a focus on the therapeutic management.
Methods: We performed an observational, multicenter, retrospective case-control study in Western Europe countries (France, Italy, Spain, Germany, Belgium). Cases were adults or children with both LVV and IBD (LVV-IBD), whereas controls had isolated TA (iTA) or GCA (iGCA).
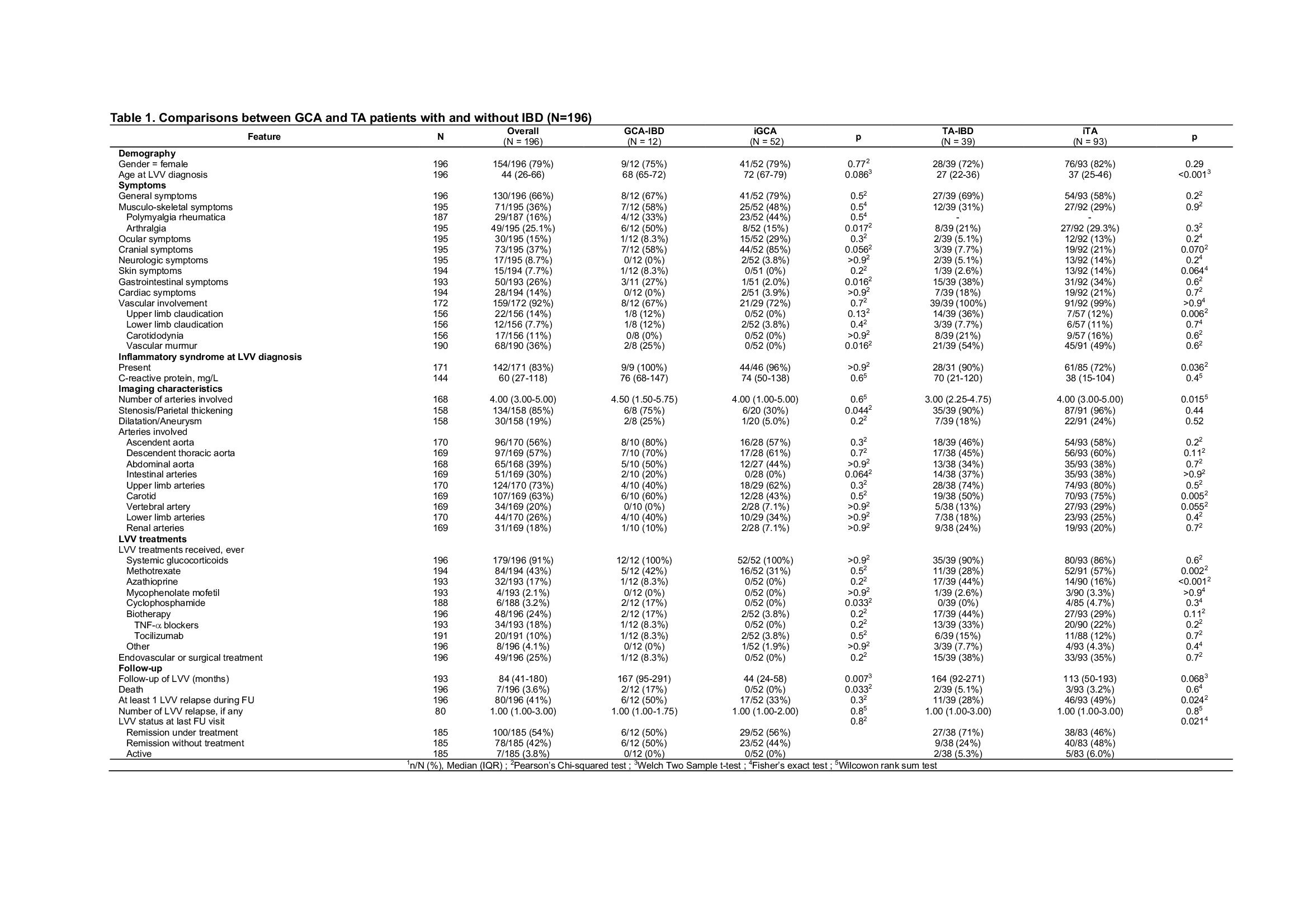
Results: Fifty-one LVV-IBD patients were included, including 39 TA-IBD and 12 GCA-IBD patients, and compared with 93 iTA and 52 iGCA. LVV-IBD patients were mostly females (75% in TA-IBD and 72% in GCA-IBD). Crohn's disease was more common in TA-IBD (67%), while ulcerative colitis was more common in GCA-IBD (58%). LVV occurred after IBD in most cases (56% in TA-IBD and 75% in GCA-IBD), with a median interval of 1 year in TA-IBD and 8.6 years in GCA-IBD, and were diagnosed concomitantly in 16%. Location, behaviour and complications of IBD were similar to previously reported IBD cohorts.
Compared to iTA patients, TA-IBD patients were significantly younger at TA diagnosis (27 vs. 37 years, p < 0.001) and had more upper limb claudication (36 vs 12%, p=0.006). The number of affected arterial segments was lower in TA-IBD (3 vs. 4 segments, p=0.015) and carotid artery involvement was less common in TA-IBD patients than in iTA patients (50 vs. 75% respectively, p=0.005).
Compared to iGCA patients, GCA-IBD patients had more arthralgia (50 vs 15%, p=0.017) and vascular murmur (25 vs 0%, p=0.016). GCA-IBD patients had more arterial thickening or stenosis than controls (75 vs. 30% respectively, p=0.044).
LVV occurred in IBD patients while on therapy in 77% (86% for TA-IBD and 56% for GCA-IBD), including oral glucocorticoids (GCs) in 36%, azathioprine (AZA) in 25%, or TNF-a blockers in 33% of TA-IBD.
LVV-IBD were treated with GCs in 91%, methotrexate in 43%, AZA in 17% or cyclophosphamide in 3.2%. Biologics were used in 24%, including mainly TNF-a blockers in TA-IBD patients (33%). AZA was used more frequently in TA-IBD patients than in iTA, with the latter mainly receiving methotrexate.
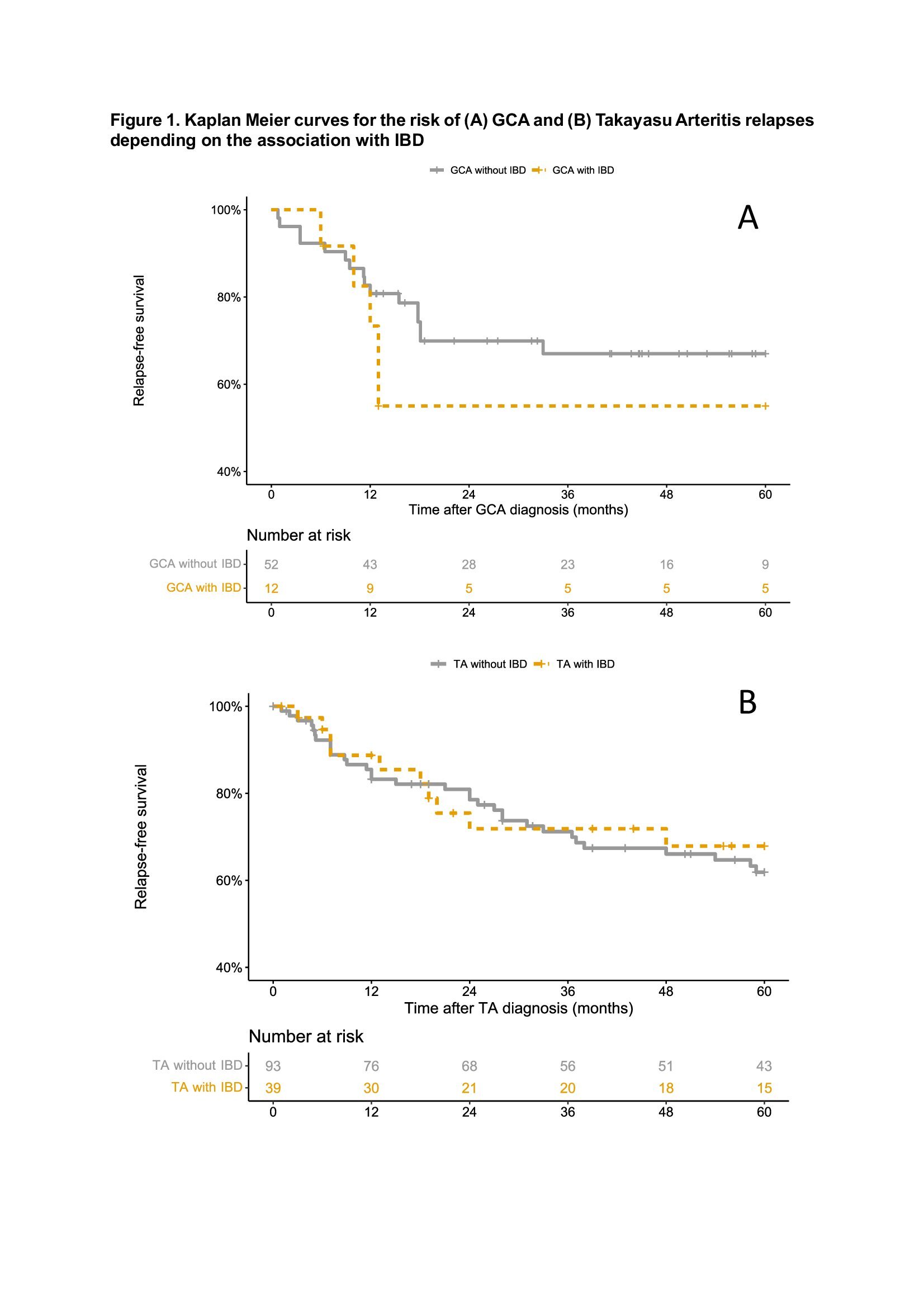
The presence of IBD did not influence the vasculitis relapse rate (OR 1.40 [0.39-5.10] for GCA and OR 0.72 [0.34-1.51] for TA). Aortic insufficiency in GCA patients (OR 8.36, 95% CI 1.35-51.66), and ascendent aorta involvement (OR 2.35, 95% CI 1.20-4.60) and the presence of general symptoms (HR 2.02, 95% CI 1.01-4.03) in TA patients were identified as independent vasculitis relapse risk factors.
Conclusion: This large case-control study identifies new clinical, imaging and outcome features in LVV-IBD. Vascular involvement seems to be less severe in TA-IBD than in iTA, while it seems to be the opposite in GCA-IBD. LVV-IBD occurred despite ongoing treatment in 77%, including AZA and/or TNF-a blockers in almost half of the cases.
F. Maillet: None; Y. Nguyen: None; O. Espitia: None; L. Perard: None; C. Salvarani: None; E. Rivière: None; C. Durel: None; P. GUILPAIN: None; L. Mouthon: None; A. Kernder: None; J. Loricera: None; P. Cohen: None; I. Melki: None; C. De Moreuil: None; N. Limal: None; A. Mekinian: None; N. Costedoat-Chalumeau: None; N. Morel: None; J. Boutemy: None; L. Raffray: None; J. Allain: None; V. Devauchelle: None; I. Kone-Paut: None; M. Fabre: None; M. Durel: None; A. Dossier: None; S. Abad: None; M. Visentini: None; A. Bigot: None; H. Yildiz: GSK, 6; O. Fain: None; M. Samson: ARGENX, 2, Boehringer-Ingelheim, 2, CHUGAI, 2, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2, NOVARTIS, 2, 5; G. Gondran: None; V. Abitbol: None; B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2.
Background/Purpose: The association of large vessel vasculitis (LVV), whether Takayasu arteritis (TA) or giant cell arteritis (GCA), with inflammatory bowel disease (IBD) is a rare and challenging condition. The prevalence of LVV in IBD cohorts is less than 1%, but the prevalence of IBD can be as high as 15.4% in TA cohorts, and population-based studies confirmed this association. We aimed to describe the characteristics and outcome of LVV-IBD patients, with a focus on the therapeutic management.
Methods: We performed an observational, multicenter, retrospective case-control study in Western Europe countries (France, Italy, Spain, Germany, Belgium). Cases were adults or children with both LVV and IBD (LVV-IBD), whereas controls had isolated TA (iTA) or GCA (iGCA).
Results: Fifty-one LVV-IBD patients were included, including 39 TA-IBD and 12 GCA-IBD patients, and compared with 93 iTA and 52 iGCA. LVV-IBD patients were mostly females (75% in TA-IBD and 72% in GCA-IBD). Crohn's disease was more common in TA-IBD (67%), while ulcerative colitis was more common in GCA-IBD (58%). LVV occurred after IBD in most cases (56% in TA-IBD and 75% in GCA-IBD), with a median interval of 1 year in TA-IBD and 8.6 years in GCA-IBD, and were diagnosed concomitantly in 16%. Location, behaviour and complications of IBD were similar to previously reported IBD cohorts.
Compared to iTA patients, TA-IBD patients were significantly younger at TA diagnosis (27 vs. 37 years, p < 0.001) and had more upper limb claudication (36 vs 12%, p=0.006). The number of affected arterial segments was lower in TA-IBD (3 vs. 4 segments, p=0.015) and carotid artery involvement was less common in TA-IBD patients than in iTA patients (50 vs. 75% respectively, p=0.005).
Compared to iGCA patients, GCA-IBD patients had more arthralgia (50 vs 15%, p=0.017) and vascular murmur (25 vs 0%, p=0.016). GCA-IBD patients had more arterial thickening or stenosis than controls (75 vs. 30% respectively, p=0.044).
LVV occurred in IBD patients while on therapy in 77% (86% for TA-IBD and 56% for GCA-IBD), including oral glucocorticoids (GCs) in 36%, azathioprine (AZA) in 25%, or TNF-a blockers in 33% of TA-IBD.
LVV-IBD were treated with GCs in 91%, methotrexate in 43%, AZA in 17% or cyclophosphamide in 3.2%. Biologics were used in 24%, including mainly TNF-a blockers in TA-IBD patients (33%). AZA was used more frequently in TA-IBD patients than in iTA, with the latter mainly receiving methotrexate.
The presence of IBD did not influence the vasculitis relapse rate (OR 1.40 [0.39-5.10] for GCA and OR 0.72 [0.34-1.51] for TA). Aortic insufficiency in GCA patients (OR 8.36, 95% CI 1.35-51.66), and ascendent aorta involvement (OR 2.35, 95% CI 1.20-4.60) and the presence of general symptoms (HR 2.02, 95% CI 1.01-4.03) in TA patients were identified as independent vasculitis relapse risk factors.
Conclusion: This large case-control study identifies new clinical, imaging and outcome features in LVV-IBD. Vascular involvement seems to be less severe in TA-IBD than in iTA, while it seems to be the opposite in GCA-IBD. LVV-IBD occurred despite ongoing treatment in 77%, including AZA and/or TNF-a blockers in almost half of the cases.


F. Maillet: None; Y. Nguyen: None; O. Espitia: None; L. Perard: None; C. Salvarani: None; E. Rivière: None; C. Durel: None; P. GUILPAIN: None; L. Mouthon: None; A. Kernder: None; J. Loricera: None; P. Cohen: None; I. Melki: None; C. De Moreuil: None; N. Limal: None; A. Mekinian: None; N. Costedoat-Chalumeau: None; N. Morel: None; J. Boutemy: None; L. Raffray: None; J. Allain: None; V. Devauchelle: None; I. Kone-Paut: None; M. Fabre: None; M. Durel: None; A. Dossier: None; S. Abad: None; M. Visentini: None; A. Bigot: None; H. Yildiz: GSK, 6; O. Fain: None; M. Samson: ARGENX, 2, Boehringer-Ingelheim, 2, CHUGAI, 2, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2, NOVARTIS, 2, 5; G. Gondran: None; V. Abitbol: None; B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2.



