Poster Session A
Infection-related rheumatic syndromes
Session: (0196–0228) Infection-related Rheumatic Disease Poster
0201: Tolerability and Safety of Recombinant Zoster Vaccine in Patients with Inflammatory Rheumatic Musculoskeletal Diseases - A Prospective Longitudinal Study over 12 Months
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Ioana Andreica, MD
Rheumazentrum Ruhrgebiet Herne
Herne, GermanyDisclosure information not submitted.
Abstract Poster Presenter(s)
Ioana Andreica1, Gianna Chierergo2, Stefania Reale2, Benjamin Wilde3, Styliani Tsiami4, David Kiefer4, Philipp Sewerin4, Hilal Kavruk5, Dimitra Karagkiozidou2, Barbara Guminski4, Andreas Kribben3, Xenofon Baraliakos6, Juergen Braun4 and Uta Kiltz7, 1Rheumazentrum Ruhrgebiet Herne, Herne, Germany, 2Rheumazentrum Ruhrgebiet, Herne, and Ruhr-Universität Bochum, Herne, Germany, 3Department of Nephrology, University Hospital Essen, University Duisburg-Essen, Essen, Germany, 4Ruhr-Universität Bochum and Rheumazentrum Ruhrgebiet, Herne, Germany, 5Rheumazentrum Ruhrgebiet, Herne, and Ruhr-Universität Bochum, Düsseldorf, Germany, 6Rheumazentrum Ruhrgebiet Herne, Ruhr-University Bochum, Herne, Germany, 7Rheumazentrum Ruhrgebiet, Herne, Germany
Background/Purpose: Herpes zoster (HZ) is common in the elderly, with a lifetime risk of 25%. The primary risk factors for HZ are advanced age and immunosuppression. The aim is to describe the safety of recombinant zoster vaccine in patients with inflammatory rheumatic and musculoskeletal diseases (RMD)
Methods: Adult patients with rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), and giant cell arteritis (GCA) were prospectively enrolled in this ongoing study. Data on demographics, vaccination, RMD diagnosis, disease activity, immunosuppressive treatments, flares, and zoster breakthrough infections were collected at months 0, 2, 3, 6 and 12. Safety assessments were performed at 2, 3, 6, and 12 months. A flare was defined as change in ASDAS ≥ 0.9 for axSpA, change in DAS-28 >1.2 for RA, or clinical signs for GCA and/or CRP ≥ 0.5 mg/dl and/or ≥30 mm. Descriptive analyses were performed
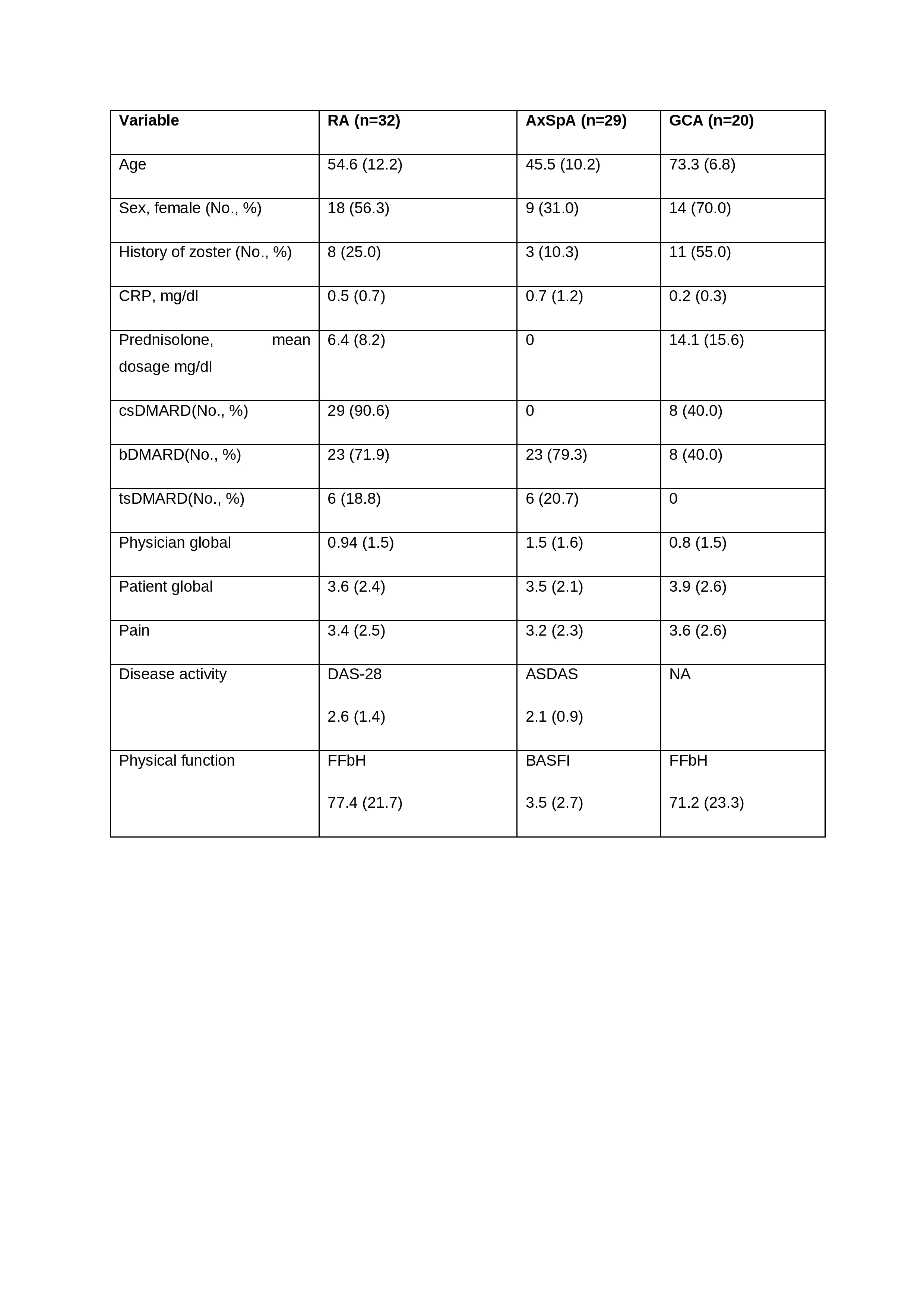
Results: 81 patients were enrolled, of whom 21 (25.9%) had a history of HZ (Table 1). All patients received RZV at month 0 and 66 patients at month 2. Safety assessments in 66, 56, 48, and 5 patients at month 2, 3, 6 and 12, respectively. A total of 87, 68, 15, 8 AEs were reported in 53, 37, 13, and 6 patients, respectively. Localized AEs (n=97 (67.0%)) were more common than generalized AEs (n=48 (33.1%)). Pain at the injection site (55 (30.9%)) was the most common AE, followed by fatigue (20 (11.2%)), musculoskeletal pain (19 (10.7%)), fever (14 (7.9%)), redness at the injection site (10 (5.6%)), and swelling at the injection site (7 (3.9%)). Serious adverse events (AE) were reported in 8 patients (3 RA, 5 GCA), none of which were vaccine-related. No patient reported an AE of special interest. 5, 4, 5 and 2 episodes of self-reported disease worsening were reported by patients at months 2, 3 and 6, respectively, 12 but none met predefined flare criteria. However, 3 patients (2 GCA, 1 RA) were hospitalized as a result. No episodes of HZ occurred during follow-up
Conclusion: Most patients tolerated RZV well with few reports of flare and serious AEs. The majority of AEs occurred within a few days of vaccination. These findings are reassuring for rheumatologists and potential vaccine recipients and support confidence in the safety of RZV in patients with RMD
I. Andreica: AbbVie/Abbott, 1, 6, Amgen, 1, 6, AstraZeneca, 1, 6, Chugai, 6, Novartis, 1, 6, Sobi, 1, 6, UCB, 1, 6; G. Chierergo: None; S. Reale: None; B. Wilde: None; S. Tsiami: None; D. Kiefer: None; P. Sewerin: AbbVie, 2, 5, 6, Biogen, 2, 6, Bristol-Myers Squibb, 2, 6, Celgene, 2, 5, 6, Chugai, 2, 5, 6, Hexal, 2, 6, Janssen-Cilag, 2, 5, 6, Lilly, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Roche, 2, 6, Sanofi-Genzyme, 2, 6, Swedish Orphan Biovitrum, 2, 6, UCB, 2, 5, 6; H. Kavruk: None; D. Karagkiozidou: None; B. Guminski: None; A. Kribben: None; X. Baraliakos: AbbVie, 2, 6, BMS, 2, 6, Chugai, 2, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Gilead, 2, 6, MSD, 2, 6, Novartis, 2, 6, Pfizer Inc, 2, 6, UCB, 2, 6; J. Braun: None; U. Kiltz: AbbVie, 2, 5, 6, Amgen, 5, Biocad, 2, 6, Biogen, 5, Bristol-Myers Squibb(BMS), 2, 5, Chugai, 2, 6, Eli Lilly, 2, 6, Fresenius, 5, Gilead, 2, 5, GlaxoSmithKline (GSK), 5, Grünenthal, 2, 6, Hexal, 5, Janssen, 2, 6, MSD, 2, 6, Novartis, 2, 5, 6, onkowiessen.de, 2, 5, Pfizer, 2, 5, 6, Roche, 2, 6, UCB, 2, 6, Viatris, 2, 5.
Background/Purpose: Herpes zoster (HZ) is common in the elderly, with a lifetime risk of 25%. The primary risk factors for HZ are advanced age and immunosuppression. The aim is to describe the safety of recombinant zoster vaccine in patients with inflammatory rheumatic and musculoskeletal diseases (RMD)
Methods: Adult patients with rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), and giant cell arteritis (GCA) were prospectively enrolled in this ongoing study. Data on demographics, vaccination, RMD diagnosis, disease activity, immunosuppressive treatments, flares, and zoster breakthrough infections were collected at months 0, 2, 3, 6 and 12. Safety assessments were performed at 2, 3, 6, and 12 months. A flare was defined as change in ASDAS ≥ 0.9 for axSpA, change in DAS-28 >1.2 for RA, or clinical signs for GCA and/or CRP ≥ 0.5 mg/dl and/or ≥30 mm. Descriptive analyses were performed
Results: 81 patients were enrolled, of whom 21 (25.9%) had a history of HZ (Table 1). All patients received RZV at month 0 and 66 patients at month 2. Safety assessments in 66, 56, 48, and 5 patients at month 2, 3, 6 and 12, respectively. A total of 87, 68, 15, 8 AEs were reported in 53, 37, 13, and 6 patients, respectively. Localized AEs (n=97 (67.0%)) were more common than generalized AEs (n=48 (33.1%)). Pain at the injection site (55 (30.9%)) was the most common AE, followed by fatigue (20 (11.2%)), musculoskeletal pain (19 (10.7%)), fever (14 (7.9%)), redness at the injection site (10 (5.6%)), and swelling at the injection site (7 (3.9%)). Serious adverse events (AE) were reported in 8 patients (3 RA, 5 GCA), none of which were vaccine-related. No patient reported an AE of special interest. 5, 4, 5 and 2 episodes of self-reported disease worsening were reported by patients at months 2, 3 and 6, respectively, 12 but none met predefined flare criteria. However, 3 patients (2 GCA, 1 RA) were hospitalized as a result. No episodes of HZ occurred during follow-up
Conclusion: Most patients tolerated RZV well with few reports of flare and serious AEs. The majority of AEs occurred within a few days of vaccination. These findings are reassuring for rheumatologists and potential vaccine recipients and support confidence in the safety of RZV in patients with RMD

Patients and disease characteristics
I. Andreica: AbbVie/Abbott, 1, 6, Amgen, 1, 6, AstraZeneca, 1, 6, Chugai, 6, Novartis, 1, 6, Sobi, 1, 6, UCB, 1, 6; G. Chierergo: None; S. Reale: None; B. Wilde: None; S. Tsiami: None; D. Kiefer: None; P. Sewerin: AbbVie, 2, 5, 6, Biogen, 2, 6, Bristol-Myers Squibb, 2, 6, Celgene, 2, 5, 6, Chugai, 2, 5, 6, Hexal, 2, 6, Janssen-Cilag, 2, 5, 6, Lilly, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Roche, 2, 6, Sanofi-Genzyme, 2, 6, Swedish Orphan Biovitrum, 2, 6, UCB, 2, 5, 6; H. Kavruk: None; D. Karagkiozidou: None; B. Guminski: None; A. Kribben: None; X. Baraliakos: AbbVie, 2, 6, BMS, 2, 6, Chugai, 2, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Gilead, 2, 6, MSD, 2, 6, Novartis, 2, 6, Pfizer Inc, 2, 6, UCB, 2, 6; J. Braun: None; U. Kiltz: AbbVie, 2, 5, 6, Amgen, 5, Biocad, 2, 6, Biogen, 5, Bristol-Myers Squibb(BMS), 2, 5, Chugai, 2, 6, Eli Lilly, 2, 6, Fresenius, 5, Gilead, 2, 5, GlaxoSmithKline (GSK), 5, Grünenthal, 2, 6, Hexal, 5, Janssen, 2, 6, MSD, 2, 6, Novartis, 2, 5, 6, onkowiessen.de, 2, 5, Pfizer, 2, 5, 6, Roche, 2, 6, UCB, 2, 6, Viatris, 2, 5.



