Poster Session A
Rheumatoid arthritis (RA)
Session: (0423–0459) RA – Treatment Poster I
0428: Real World Patterns of Advanced Therapy Tapering in Early Rheumatoid Arthritis: Data from the Canadian Early Arthritis Cohort
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MP
Maria Powell, MD
University of Calgary
Calgary, AB, CanadaDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Maria Powell1, Vivian Bykerk2, Orit Schieir3, Marie-France Valois4, Susan Bartlett5, Louis Bessette6, Gilles Boire7, Carol Hitchon8, Edward Keystone9, Janet Pope10, Carter Thorne11, Diane Tin12, Glen Hazlewood1 and Canadian Early Arthritis Cohort (CATCH) Investigators13, 1University of Calgary, Calgary, AB, Canada, 2Hospital for Special Surgery, New York, NY, 3McGill University, Montréal, QC, Canada, 4McGill University, Pointe-Claire, QC, Canada, 5McGill University, Montreal, QC, Canada, 6Centre de l'Ostéoporose et de Rhumatologie de Québec, Quebec City, QC, Canada, 7Université de Sherbrooke, Sherbrooke, QC, Canada, 8University of Manitoba, Manitoba, MB, Canada, 9Keystone Consulting Enterprises Inc., Toronto, ON, Canada, 10University of Western Ontario, London, ON, Canada, 11Southlake Regional Health Centre, Newmarket, ON, Canada, 12Centre of Arthritis Excellence, Newmarket, ON, Canada, 13CATCH, Toronto, ON, Canada
Background/Purpose: Recent Canadian treatment recommendations suggest offering to taper of biologic or targeted synthetic therapy to patients with rheumatoid arthritis (RA) after they achieve sustained remission (sREM) or low disease activity (LDA) for at least 6 months. This study sought to describe real-world patterns of advanced therapy tapering in a large pan-Canadian cohort of patients with early RA who would be eligible to taper and identify predictors of which patients are reducing their therapy in routine care.
Methods: Data from patients (age >18) enrolled in the Canadian early ArThritis CoHort (CATCH) between January 2007 to August 2022 were analyzed. CATCH is a prospective, observational cohort of patients with early inflammatory arthritis (symptoms < 1 year) treated in rheumatology clinics across Canada. The analysis cohort included patients with a diagnosis of RA according to the 1987 or 2010 ACR/EULAR classification criteria who were prescribed advanced therapy (i.e., tumour necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, non-TNF inhibitor advanced therapy) and sREM or LDA (using simplified disease activity index or clinical disease activity index) for at least 6 months. Patients were considered taper advanced therapy if they reduced the dose of therapy by at least 25% through dose reduction or dose spacing for a time period of at least 3 months. Survival analysis using the Kaplan-Meier method was used to estimate the median time to taper advanced therapy and Cox regression analysis was used to identify predictors of tapering advanced therapy.
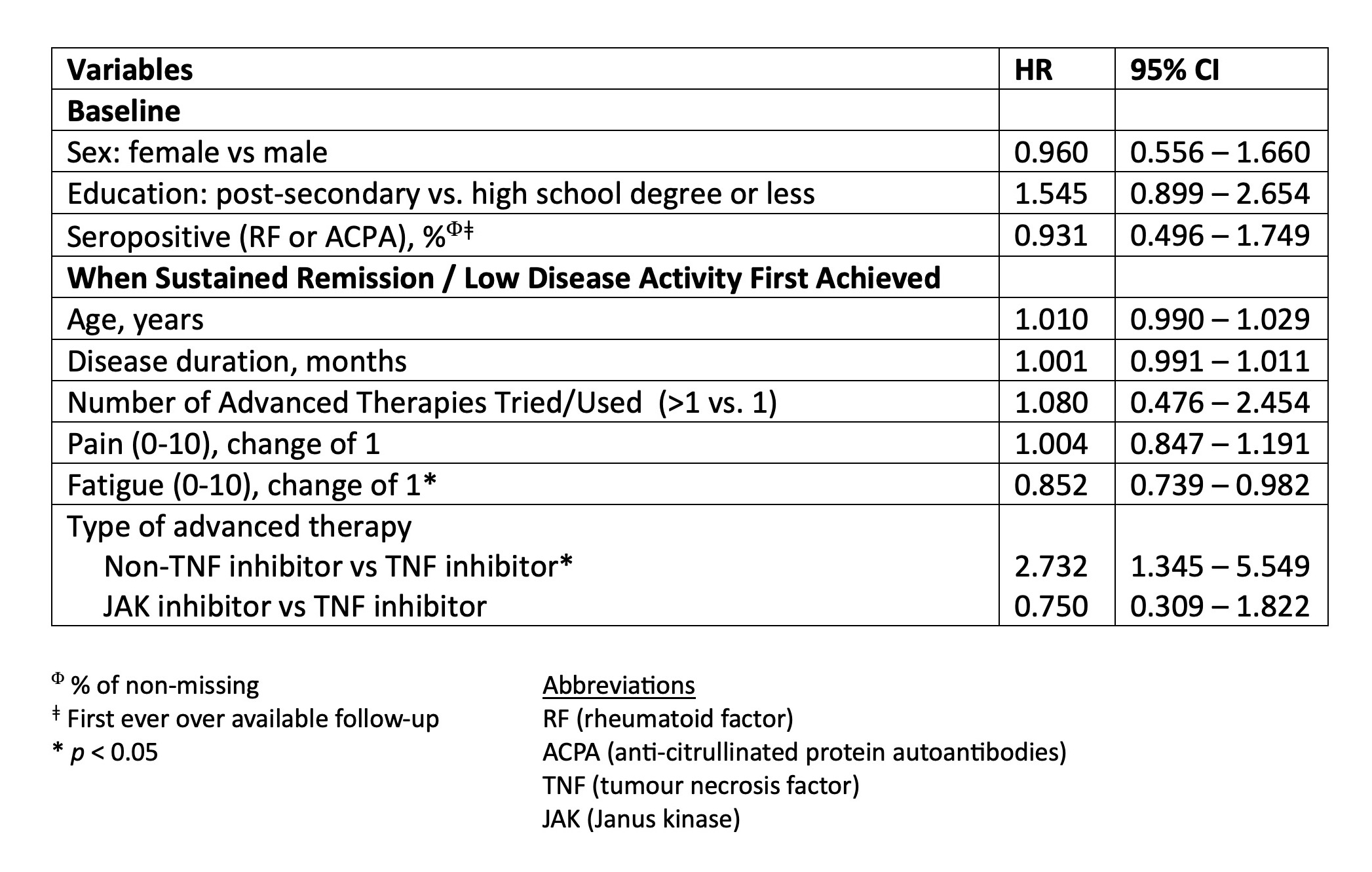
Results: Data were analyzed from 306 RA patients treated with advanced therapy who reached sREM or LDA for at least 6 months. These patients were predominantly white (87%), female (73%), and had seropositive RA (82%) with a mean age of 50 years. At the time of sREM or LDA patients were treated with TNF inhibitors [n=218 (71%)], JAK inhibitors [n=52 (17%)], and non-TNF biologic therapy [n=36 (12%)]. Treatment tapering occurred in 72 (24%) patients and was more frequent for treatment with non-TNF-inhibitors [n=15/36 (42%)] compared to TNF inhibitors [n=51/218 (23%)] or JAK inhibitors [n=6/52 (12%)]. The median time to taper advanced therapy after achieving sREM or LDA was 14 months (IQR: 5, 35 months) (Figure 1). In adjusted Cox regression analyses, treatment with a non-TNF inhibitor was associated with a higher likelihood of tapering (HR: 2.73, 95% CI:1.35, 5.55) and having higher patient reported symptoms of fatigue was associated with a decreased likelihood of tapering (HR 0.89: 95% CI 0.80 – 0.99) (Table 1).
Conclusion: In this real-world study of early RA patients, tapering was attempted in approximately 1 in 4 patients who would be considered eligible based on current recommendations, with a median time of 14 months. Tapering was associated with the type of advanced therapy and was less common in patients with worse fatigue, suggesting real-world decisions to taper are influenced by factors beyond typical measures of RA disease activity.
.jpg)
M. Powell: None; V. Bykerk: Abbvie, 2, BMS, 2, Pfizer, 2; O. Schieir: None; M. Valois: None; S. Bartlett: Janssen, 6, Merck/MSD, 2, 6, Novartis, 2, Organon, 1, 6, PROMIS Health Organization, 4, Sandoz, 2, 6; L. Bessette: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Bristol Myers Squibb, 2, 5, 6, Eli Lilly, 2, 5, 6, Fresenius Kabi, 2, 6, Gilead, 2, 5, 6, JAMP Pharma, 2, 5, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Organon, 2, 6, Pfizer, 2, 5, 6, Sandoz, 2, 6, Sanofi, 2, 5, 6, Teva, 2, 6, UCB, 2, 5, 6, UCBA, 5; G. Boire: Eli Lilly, 1, Janssen, 6, Organon, 1, Orimed Pharma, 1, 6, Otsuka, 1, Pfizer, 1, 5, Sandoz, 1, Teva, 1, Viatris, 1, 6; C. Hitchon: Astra Zeneca, 1, Pfizer Canada, 5; E. Keystone: AbbVie/Abbott, 2, 6, Amgen, 2, 6, celltrion, 2, 6, Eli Lilly, 2, 6, Fresenius Kabi, 2, 6, Pfizer, 2, 6, Samsung Bioepsis, 2, sandoz, 2, 6; J. Pope: AbbVie, 1, 2; C. Thorne: Abbvie, 1, Biogen, 2, Nordic Pharma, 1, Pfizer, 1, 5, Roche, 1, Sandoz, 1, 2; D. Tin: None; G. Hazlewood: None; C. (CATCH) Investigators: None.
Background/Purpose: Recent Canadian treatment recommendations suggest offering to taper of biologic or targeted synthetic therapy to patients with rheumatoid arthritis (RA) after they achieve sustained remission (sREM) or low disease activity (LDA) for at least 6 months. This study sought to describe real-world patterns of advanced therapy tapering in a large pan-Canadian cohort of patients with early RA who would be eligible to taper and identify predictors of which patients are reducing their therapy in routine care.
Methods: Data from patients (age >18) enrolled in the Canadian early ArThritis CoHort (CATCH) between January 2007 to August 2022 were analyzed. CATCH is a prospective, observational cohort of patients with early inflammatory arthritis (symptoms < 1 year) treated in rheumatology clinics across Canada. The analysis cohort included patients with a diagnosis of RA according to the 1987 or 2010 ACR/EULAR classification criteria who were prescribed advanced therapy (i.e., tumour necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, non-TNF inhibitor advanced therapy) and sREM or LDA (using simplified disease activity index or clinical disease activity index) for at least 6 months. Patients were considered taper advanced therapy if they reduced the dose of therapy by at least 25% through dose reduction or dose spacing for a time period of at least 3 months. Survival analysis using the Kaplan-Meier method was used to estimate the median time to taper advanced therapy and Cox regression analysis was used to identify predictors of tapering advanced therapy.
Results: Data were analyzed from 306 RA patients treated with advanced therapy who reached sREM or LDA for at least 6 months. These patients were predominantly white (87%), female (73%), and had seropositive RA (82%) with a mean age of 50 years. At the time of sREM or LDA patients were treated with TNF inhibitors [n=218 (71%)], JAK inhibitors [n=52 (17%)], and non-TNF biologic therapy [n=36 (12%)]. Treatment tapering occurred in 72 (24%) patients and was more frequent for treatment with non-TNF-inhibitors [n=15/36 (42%)] compared to TNF inhibitors [n=51/218 (23%)] or JAK inhibitors [n=6/52 (12%)]. The median time to taper advanced therapy after achieving sREM or LDA was 14 months (IQR: 5, 35 months) (Figure 1). In adjusted Cox regression analyses, treatment with a non-TNF inhibitor was associated with a higher likelihood of tapering (HR: 2.73, 95% CI:1.35, 5.55) and having higher patient reported symptoms of fatigue was associated with a decreased likelihood of tapering (HR 0.89: 95% CI 0.80 – 0.99) (Table 1).
Conclusion: In this real-world study of early RA patients, tapering was attempted in approximately 1 in 4 patients who would be considered eligible based on current recommendations, with a median time of 14 months. Tapering was associated with the type of advanced therapy and was less common in patients with worse fatigue, suggesting real-world decisions to taper are influenced by factors beyond typical measures of RA disease activity.
.jpg)
Figure 1: Kaplan Meier Survival Estimates of Time from Rheumatoid Arthritis Sustained Remission or Low Disease Activity to First Advanced Therapy Taper in a Multi-Centre Study Embedded in Routine Care (72 tapered and 234 censored).

Table 1: Multivariable Cox Regression Results - Predictors of Tapering Advanced Therapy in Rheumatoid Arthritis Patients Reaching Sustained Remission or Low Disease Activity in a Multi-Centre Study Embedded in Routine Care (N=306).
M. Powell: None; V. Bykerk: Abbvie, 2, BMS, 2, Pfizer, 2; O. Schieir: None; M. Valois: None; S. Bartlett: Janssen, 6, Merck/MSD, 2, 6, Novartis, 2, Organon, 1, 6, PROMIS Health Organization, 4, Sandoz, 2, 6; L. Bessette: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Bristol Myers Squibb, 2, 5, 6, Eli Lilly, 2, 5, 6, Fresenius Kabi, 2, 6, Gilead, 2, 5, 6, JAMP Pharma, 2, 5, 6, Janssen, 2, 5, 6, Novartis, 2, 5, 6, Organon, 2, 6, Pfizer, 2, 5, 6, Sandoz, 2, 6, Sanofi, 2, 5, 6, Teva, 2, 6, UCB, 2, 5, 6, UCBA, 5; G. Boire: Eli Lilly, 1, Janssen, 6, Organon, 1, Orimed Pharma, 1, 6, Otsuka, 1, Pfizer, 1, 5, Sandoz, 1, Teva, 1, Viatris, 1, 6; C. Hitchon: Astra Zeneca, 1, Pfizer Canada, 5; E. Keystone: AbbVie/Abbott, 2, 6, Amgen, 2, 6, celltrion, 2, 6, Eli Lilly, 2, 6, Fresenius Kabi, 2, 6, Pfizer, 2, 6, Samsung Bioepsis, 2, sandoz, 2, 6; J. Pope: AbbVie, 1, 2; C. Thorne: Abbvie, 1, Biogen, 2, Nordic Pharma, 1, Pfizer, 1, 5, Roche, 1, Sandoz, 1, 2; D. Tin: None; G. Hazlewood: None; C. (CATCH) Investigators: None.



