Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Animal Models (2443–2448)
2448: UV Light Exposure Induces a Type I Interferon Dependent Activation and Migration of Inflammatory Dendritic Cells to Local Lymph Nodes
Tuesday, November 14, 2023
3:15 PM - 3:25 PM PT
Location: Room 26A-B
- XS
Xizhang Sun, PhD
University of Washington
Seattle, WA, United StatesDisclosure information not submitted.
Presenting Author(s)
Xizhang Sun, Jaime Chao, Michael Gerner and Keith Elkon, University of Washington, Seattle, WA
Background/Purpose: Photosensitivity occurs in ~ 75% of lupus patients. Although ultraviolet (UV) light stimulates Type I interferon (IFN-I) in the skin, why lupus patients are sensitive to UV skin injury and how systemic immune responses are generated are poorly understood. Following skin inflammation in other contexts, DC migrate to draining lymph nodes (dLN) and activate adaptive immune cells. In virus infections that induce Type I interferon (IFN-I), a unique inflammatory DC subset (infl-cDC2) that are potent antigen presenting cells are generated. To explore the links between UV mediated skin inflammation and innate responses, we examined myeloid cell migration, including DC subsets (cDC1, cDC2, infl-cDC2) to dLNs in normal and a lupus prone mouse strain. We also examined the influence of Type-I IFN in migratory DC differentiation.
Methods: Mice were females aged 8-12 weeks of the strains C57BL/6 (B6); KiKR (photoactivatable); interferon receptor deficient (Ifnar KO); and Trex1 mutant mice (knock in of the lupus mutant allele, D18N, Trex1m). Mice were exposed to a single dose of UV (500mJ/cm2) on the dorsal skin. In some experiments, the skin was painted with TRITC prior to UV to determine the origin of cells in the dLN. Brachial and axillary dLN were harvested at 1- or 2-days post UV exposure. Flow cytometry using the Symphony A3 cytometer identified myeloid cells as described previously. Statistical significance between groups was determined by Student's t-test.
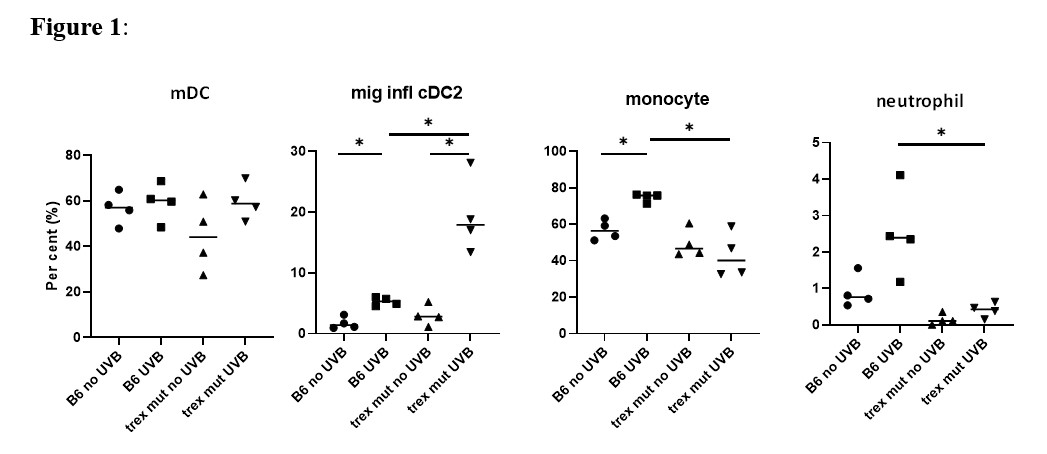
Results: Skin painted with TRITC confirmed that skin derived DC migrated to dLN after UV. When compared to non-UV exposed mice and control non-draining LN, UV caused an increase in TRITC+CD64+ and TRITC+ Ly6Ghi (neutrophils) cells in dLN. UV of KiKR mouse skin revealed that both cDC1 and cDC2 cells migrated to dLN following UV. Higher numbers of neutrophils as well as CD64+ myeloid cells, that included monocytes as well as infl-cDC2 (CD26+MAR-1+), were detected in the dLN after UV, compared to non-UV exposed control mice (Fig.1). We observed a significant reduction of infl-cDC2 cells in Ifnar KO mice indicating that their development was IFN-I dependent. Finally, we quantified myeloid cell populations in the dLN of lupus-prone Trex1m mice. Although UV did not increase cDC1 nor cDC2 in dLN, we observed a significant increase in the infl-cDC2 in the dLN in this lupus prone strain (Fig.1). Curiously, monocyte and neutrophil migration to dLN were reduced in Trex1m mice compared to B6 after UV.
Conclusion: We conclude that exposure of normal skin to UV light causes migration of many different types of myeloid cells to dLN, including skin-derived migratory DC, neutrophils, and monocytes. A subset of CD64+ infl-cDC2 is also induced and is dependent on UV-induced IFN-I. Of interest, Trex1m mice exhibit altered myeloid cell responses in the dLNs, including increased representation of infl-DCs and reduced neutrophil and monocyte cellularity. Overall, these findings show marked and non-equivalent IFN-I dependent effects of UV on myeloid cell responses in the dLN in wild type vs lupus-prone mice, implicating a link between IFN-I and the development of APCs that promote the adaptive immune response.
X. Sun: None; J. Chao: None; M. Gerner: None; K. Elkon: None.
Background/Purpose: Photosensitivity occurs in ~ 75% of lupus patients. Although ultraviolet (UV) light stimulates Type I interferon (IFN-I) in the skin, why lupus patients are sensitive to UV skin injury and how systemic immune responses are generated are poorly understood. Following skin inflammation in other contexts, DC migrate to draining lymph nodes (dLN) and activate adaptive immune cells. In virus infections that induce Type I interferon (IFN-I), a unique inflammatory DC subset (infl-cDC2) that are potent antigen presenting cells are generated. To explore the links between UV mediated skin inflammation and innate responses, we examined myeloid cell migration, including DC subsets (cDC1, cDC2, infl-cDC2) to dLNs in normal and a lupus prone mouse strain. We also examined the influence of Type-I IFN in migratory DC differentiation.
Methods: Mice were females aged 8-12 weeks of the strains C57BL/6 (B6); KiKR (photoactivatable); interferon receptor deficient (Ifnar KO); and Trex1 mutant mice (knock in of the lupus mutant allele, D18N, Trex1m). Mice were exposed to a single dose of UV (500mJ/cm2) on the dorsal skin. In some experiments, the skin was painted with TRITC prior to UV to determine the origin of cells in the dLN. Brachial and axillary dLN were harvested at 1- or 2-days post UV exposure. Flow cytometry using the Symphony A3 cytometer identified myeloid cells as described previously. Statistical significance between groups was determined by Student's t-test.
Results: Skin painted with TRITC confirmed that skin derived DC migrated to dLN after UV. When compared to non-UV exposed mice and control non-draining LN, UV caused an increase in TRITC+CD64+ and TRITC+ Ly6Ghi (neutrophils) cells in dLN. UV of KiKR mouse skin revealed that both cDC1 and cDC2 cells migrated to dLN following UV. Higher numbers of neutrophils as well as CD64+ myeloid cells, that included monocytes as well as infl-cDC2 (CD26+MAR-1+), were detected in the dLN after UV, compared to non-UV exposed control mice (Fig.1). We observed a significant reduction of infl-cDC2 cells in Ifnar KO mice indicating that their development was IFN-I dependent. Finally, we quantified myeloid cell populations in the dLN of lupus-prone Trex1m mice. Although UV did not increase cDC1 nor cDC2 in dLN, we observed a significant increase in the infl-cDC2 in the dLN in this lupus prone strain (Fig.1). Curiously, monocyte and neutrophil migration to dLN were reduced in Trex1m mice compared to B6 after UV.
Conclusion: We conclude that exposure of normal skin to UV light causes migration of many different types of myeloid cells to dLN, including skin-derived migratory DC, neutrophils, and monocytes. A subset of CD64+ infl-cDC2 is also induced and is dependent on UV-induced IFN-I. Of interest, Trex1m mice exhibit altered myeloid cell responses in the dLNs, including increased representation of infl-DCs and reduced neutrophil and monocyte cellularity. Overall, these findings show marked and non-equivalent IFN-I dependent effects of UV on myeloid cell responses in the dLN in wild type vs lupus-prone mice, implicating a link between IFN-I and the development of APCs that promote the adaptive immune response.

Fig 1. Percentage of cells in the draining Lymph Nodes (dLN). B6 or Trex1 mutant (mut) mice were exposed to 500 mJ/cm2 UV light on the dorsum. 48 hours after UV exposure, the brachial and axillary LN were harvested. The dissociated cells were analyzed by flow cytometry using 15 markers to distinguish different myeloid population on the Symphony A3 cytometer. The percentage of each cell type was calculated and comparisons made by Student’s t test. mDC = migratory DC. * p<0.05.
X. Sun: None; J. Chao: None; M. Gerner: None; K. Elkon: None.



