Poster Session A
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (0283–0307) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
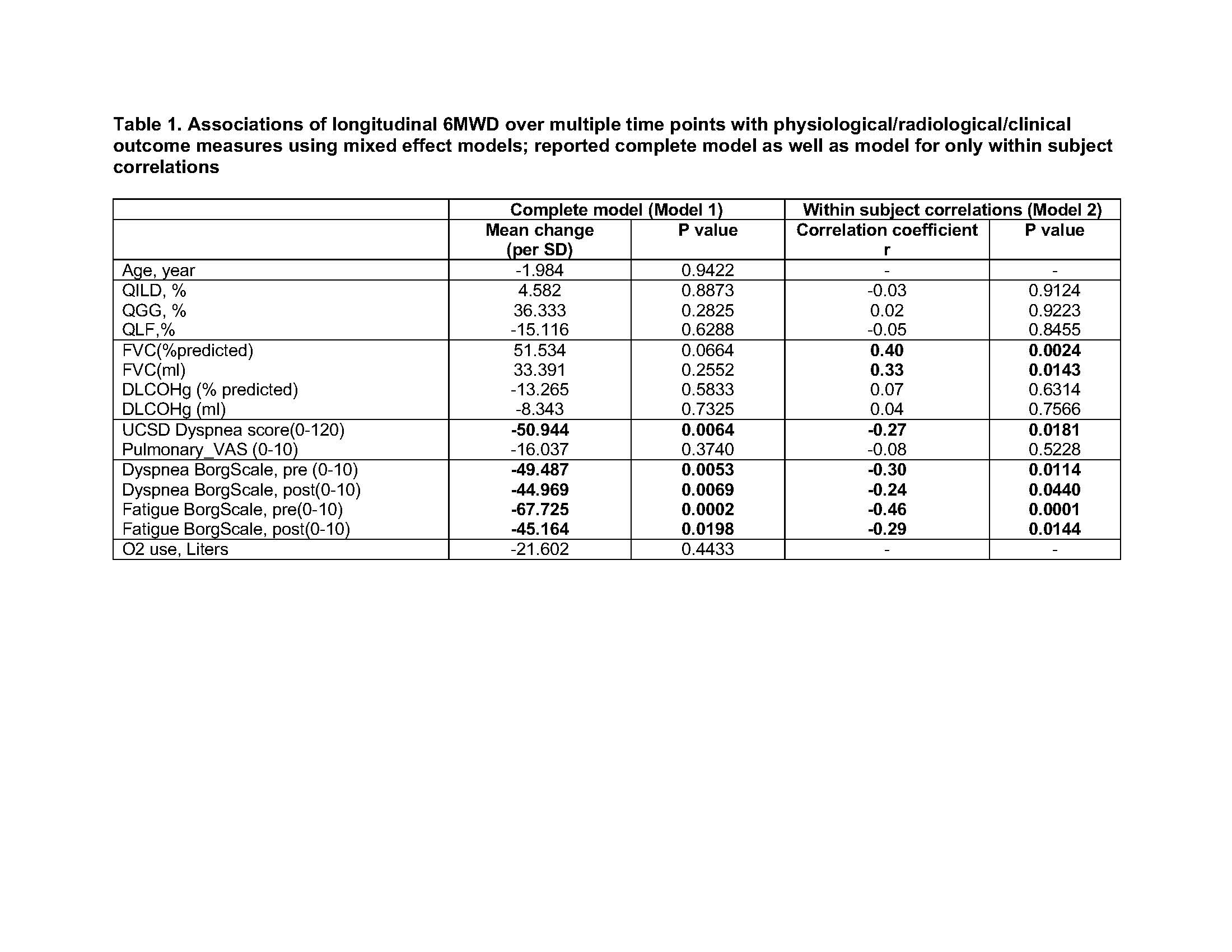
0287: 6-Minute Walk Distance Associates with Physiologic Measures and Physician/Patient Reported Outcomes in Myositis Related Interstitial Lung Disease
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
Abstract Poster Presenter(s)
Sangmee Bae1, Fereidoun Abtin2, Grace Kim3, Daniela Markovic4, Siamak Moghadam-Kia5, Chester V. Oddis6, Lila Pourzand2, Didem Saygin6, Daniel Sullivan7, Koichi Yamaguchi8, Donald Tashkin3, Christina Charles-Schoeman9, Jonathan Goldin3 and Rohit Aggarwal6, 1UCLA Rheumatology, Los Angeles, CA, 2UCLA Radiological Sciences, Los Angeles, CA, 3University of California Los Angeles, Los Angeles, CA, 4UCLA Department of Medicine Statistics Core, Los Angeles, CA, 5University of Pittsburgh Medical Center, Pittsburgh, PA, 6University of Pittsburgh, Pittsburgh, PA, 7UCLA Pulmonology, Pittsburgh, PA, 8University of Pittsburgh Medical Center Rheumatology, Pittsburgh, PA, 9UCLA Medical Center, Santa Monica, CA
Background/Purpose: The 6 minute walk distance (6MWD) provides a global evaluation of sub-maximal exercise capacity that is easily performed and highly reproducible, and is used as an outcome measure for ILD. We evaluated the association of 6MWD with physiological, clinical and radiographic measures of interstitial lung disease (ILD) as well as the responsiveness to change in patients with myositis-ILD.
Methods: We analyzed 20 patients enrolled in the Abatacept in Myositis Associated Interstitial lung disease (Attack My-ILD) study. Myositis patients with antisynthetase antibodies and active ILD were enrolled across 5 centers for 24 weeks followed by an open label period for 24 weeks. 6MWD, pulmonary function tests (forced vital capacity; FVC, diffusing capacity adjusted for hemoglobin; DLCOHg), physician/patient reported outcomes (UCSD shortness of breath questionnaire, Borg scale, pulmonary visual analog scale as part of the myositis disease activity assessment tool, supplemental oxygen use) were obtained over 5 timepoints (wk 0, 12, 24, 36, 48) and high resolution CT images were done at 3 timepoints (wk 0, 24, 48) with quantitative scores for extent of ground glass (QGG), fibrosis (QLF), total ILD (QILD) performed using a computer aided diagnostic system previously used in scleroderma ILD. Mixed effect models were used to estimate the associations between 6MWD and various physiologic, clinical and radiographic parameters longitudinally over multiple time points.
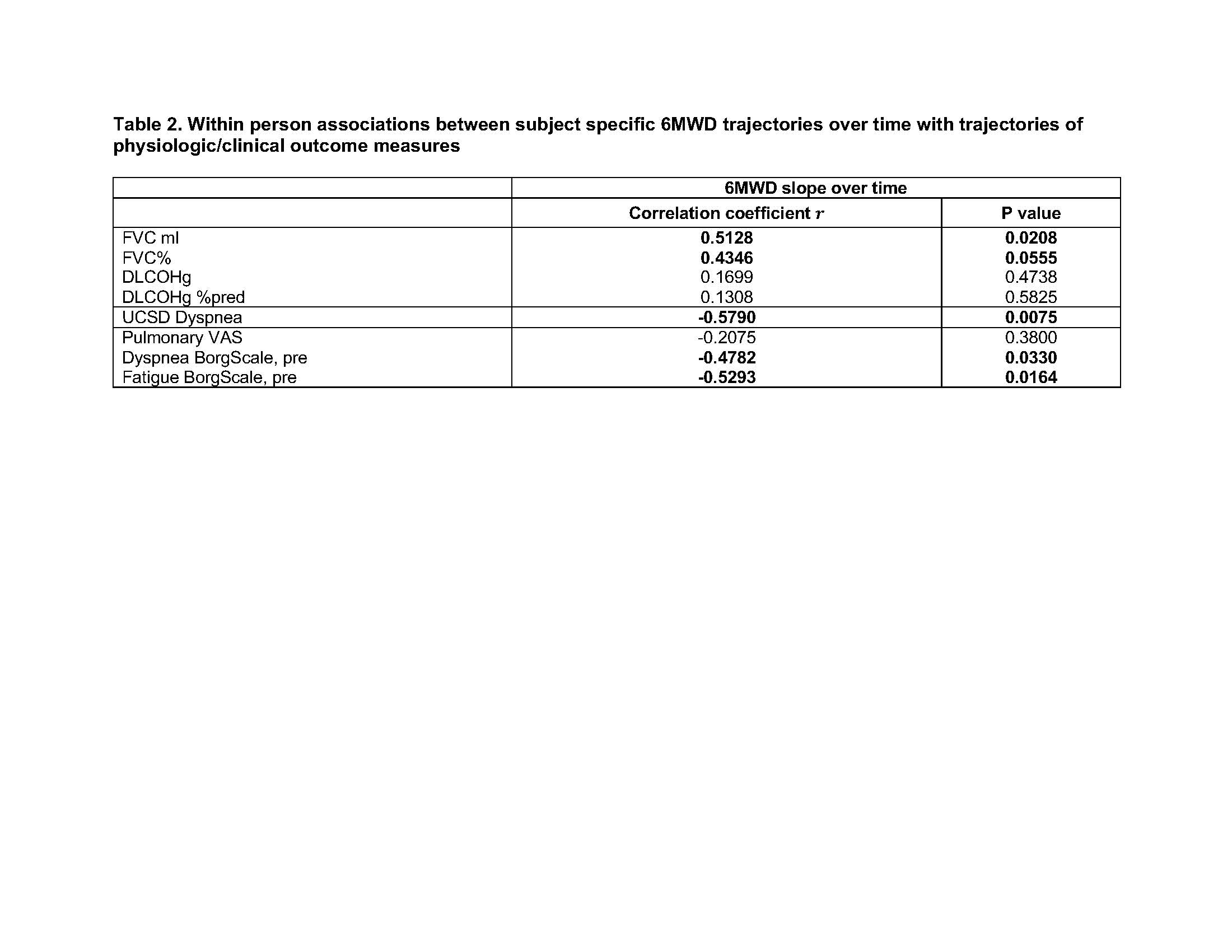
Results: Shorter 6MWD was correlated with greater extent of fibrosis on HRCT (QLF) at baseline (r=-0.54, p=0.03) while other variables including physician and patient global myositis disease activity scores, MMT, muscle enzymes, HAQ and SF-36 were not correlated with 6MWD at baseline. Longitudinal 6MWD over multiple timepoints estimated by mixed effect models was significantly correlated with worse longitudinal dyspnea score by the UCSD shortness of breath questionnaire, and worse dyspnea and fatigue on the Borg scale (Table 1). Within each subject, longitudinal 6MWD was positively correlated with longitudinal FVC (Table 1, model 2), although when considering both within subject and between subject effects the correlation was no longer significant (Table 1, model 1). Change in 6MWD from baseline to each time point had significant positive correlations with FVC change from baseline as well as with pre-test Borg scale change from baseline, and similar trends were seen for changes in pulmonary VAS and changes in dyspnea score (Figure 1). We used mixed effect models to estimate the trajectories (slopes) of the 6MWD over time for each subject and found estimated slopes correlated with slopes for FVC, dyspnea score and pretest Borg scales for dyspnea and fatigue (Table 2).
Conclusion: Longitudinal measurements of 6MWD reflected parallel changes in FVC as well as patient and physician reported outcomes in patients with myositis ILD.
.jpg)
S. Bae: None; F. Abtin: None; G. Kim: MedQIA, 2; D. Markovic: None; S. Moghadam-Kia: None; C. V. Oddis: Boehringer-Ingelheim, 5, Cabaletta, 5, EMD Serono, 5, Novartis, 5, Pfizer, 1; L. Pourzand: None; D. Saygin: None; D. Sullivan: None; K. Yamaguchi: None; D. Tashkin: None; C. Charles-Schoeman: AbbVie, 2, 5, Alexion, 5, BMS, 2, 5, Boehringer Ingleheim, 2, 5, CSL Behring, 5, Galapagos, 2, Pfizer, 2, 5, Priovant, 2, 5, Recludix, 2; J. Goldin: MedQIA, 12, Founder; R. Aggarwal: Actigraph, 2, Alexion, 2, ANI Pharmaceuticals, 2, Argenx, 2, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, CabalettaBio, 2, Capella Bioscience, 2, Corbus, 2, CSL Behring, 2, EMD Serono, 2, 5, Galapagos, 2, Horizon Therapeutics, 2, I-Cell, 2, Janssen, 2, 5, Kezar, 2, Kyverna, 2, Mallinckrodt, 5, Merck, 2, Octapharma, 2, Pfizer, 2, 5, Q32, 5, Roivant, 2, Sanofi, 2, Teva, 2.
Background/Purpose: The 6 minute walk distance (6MWD) provides a global evaluation of sub-maximal exercise capacity that is easily performed and highly reproducible, and is used as an outcome measure for ILD. We evaluated the association of 6MWD with physiological, clinical and radiographic measures of interstitial lung disease (ILD) as well as the responsiveness to change in patients with myositis-ILD.
Methods: We analyzed 20 patients enrolled in the Abatacept in Myositis Associated Interstitial lung disease (Attack My-ILD) study. Myositis patients with antisynthetase antibodies and active ILD were enrolled across 5 centers for 24 weeks followed by an open label period for 24 weeks. 6MWD, pulmonary function tests (forced vital capacity; FVC, diffusing capacity adjusted for hemoglobin; DLCOHg), physician/patient reported outcomes (UCSD shortness of breath questionnaire, Borg scale, pulmonary visual analog scale as part of the myositis disease activity assessment tool, supplemental oxygen use) were obtained over 5 timepoints (wk 0, 12, 24, 36, 48) and high resolution CT images were done at 3 timepoints (wk 0, 24, 48) with quantitative scores for extent of ground glass (QGG), fibrosis (QLF), total ILD (QILD) performed using a computer aided diagnostic system previously used in scleroderma ILD. Mixed effect models were used to estimate the associations between 6MWD and various physiologic, clinical and radiographic parameters longitudinally over multiple time points.
Results: Shorter 6MWD was correlated with greater extent of fibrosis on HRCT (QLF) at baseline (r=-0.54, p=0.03) while other variables including physician and patient global myositis disease activity scores, MMT, muscle enzymes, HAQ and SF-36 were not correlated with 6MWD at baseline. Longitudinal 6MWD over multiple timepoints estimated by mixed effect models was significantly correlated with worse longitudinal dyspnea score by the UCSD shortness of breath questionnaire, and worse dyspnea and fatigue on the Borg scale (Table 1). Within each subject, longitudinal 6MWD was positively correlated with longitudinal FVC (Table 1, model 2), although when considering both within subject and between subject effects the correlation was no longer significant (Table 1, model 1). Change in 6MWD from baseline to each time point had significant positive correlations with FVC change from baseline as well as with pre-test Borg scale change from baseline, and similar trends were seen for changes in pulmonary VAS and changes in dyspnea score (Figure 1). We used mixed effect models to estimate the trajectories (slopes) of the 6MWD over time for each subject and found estimated slopes correlated with slopes for FVC, dyspnea score and pretest Borg scales for dyspnea and fatigue (Table 2).
Conclusion: Longitudinal measurements of 6MWD reflected parallel changes in FVC as well as patient and physician reported outcomes in patients with myositis ILD.

Model estimate parameters for model 1 are mean change per standard deviation of the specified units and correlation coefficients for model 2. Model 2 was calculated by conditioning between person effects as a fixed effect.
Abbreviations: QILD, quantitative ILD score on HRCT; QGG, quantitative ground glass score on HRCT; QLF, quantitative fibrosis score on HRCT; FVC, forced vital capacity, DLCO Hg, diffusing capacity adjusted by hemoglobin; UCSD Dyspnea score, University of California San Diego Shortness of Breath Questionnaire with higher scores for worse dyspnea; Pulmonary VAS, pulmonary visual analog scale from the Myositis disease activity assessment tool (MDAAT); Borg scale, Borg scale for dyspnea and fatigue before and after test with higher score indicating more severe symptoms.
Abbreviations: QILD, quantitative ILD score on HRCT; QGG, quantitative ground glass score on HRCT; QLF, quantitative fibrosis score on HRCT; FVC, forced vital capacity, DLCO Hg, diffusing capacity adjusted by hemoglobin; UCSD Dyspnea score, University of California San Diego Shortness of Breath Questionnaire with higher scores for worse dyspnea; Pulmonary VAS, pulmonary visual analog scale from the Myositis disease activity assessment tool (MDAAT); Borg scale, Borg scale for dyspnea and fatigue before and after test with higher score indicating more severe symptoms.
.jpg)
Blue line represents the estimated correlation between percent changes using mixed effect models. Each colored line represents percent change from baseline of each parameter in each subject at multiple time points.

Subject specific trajectories over time estimated using the mixed model and correlations between slopes computed using Spearman’s correlation
S. Bae: None; F. Abtin: None; G. Kim: MedQIA, 2; D. Markovic: None; S. Moghadam-Kia: None; C. V. Oddis: Boehringer-Ingelheim, 5, Cabaletta, 5, EMD Serono, 5, Novartis, 5, Pfizer, 1; L. Pourzand: None; D. Saygin: None; D. Sullivan: None; K. Yamaguchi: None; D. Tashkin: None; C. Charles-Schoeman: AbbVie, 2, 5, Alexion, 5, BMS, 2, 5, Boehringer Ingleheim, 2, 5, CSL Behring, 5, Galapagos, 2, Pfizer, 2, 5, Priovant, 2, 5, Recludix, 2; J. Goldin: MedQIA, 12, Founder; R. Aggarwal: Actigraph, 2, Alexion, 2, ANI Pharmaceuticals, 2, Argenx, 2, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, CabalettaBio, 2, Capella Bioscience, 2, Corbus, 2, CSL Behring, 2, EMD Serono, 2, 5, Galapagos, 2, Horizon Therapeutics, 2, I-Cell, 2, Janssen, 2, 5, Kezar, 2, Kyverna, 2, Mallinckrodt, 5, Merck, 2, Octapharma, 2, Pfizer, 2, 5, Q32, 5, Roivant, 2, Sanofi, 2, Teva, 2.




