Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Treatment II: Nonrenal (2485–2490)
2488: Efficacy and Safety of ABBV-599 High Dose (Elsubrutinib 60 mg and Upadacitinib 30 mg) and Upadacitinib Monotherapy for the Treatment of Systemic Lupus Erythematosus: A Phase 2, Double-blind, Placebo-controlled Trial
Tuesday, November 14, 2023
2:45 PM - 2:55 PM PT
Location: Ballroom 20D
- JM
Joan Merrill, MD
Oklahoma Medical Research Foundation
Oklahoma City 73104, OK, United StatesDisclosure information not submitted.
Presenting Author(s)
Joan Merrill1, Yoshiya Tanaka2, David D'Cruz3, Karina Vila-Rivera4, Daniel Siri5, Xiaofeng Zeng6, Kristin D'Silva7, Ling Cheng7, Thierry Sornasse7, Thao Doan7, Denise Kruzikas7 and Alan Friedman7, 1Oklahoma Medical Research Foundation, Oklahoma City, OK, 2University of Occupational and Environmental Health, Kitakyushu, Japan, 3King's College London, London, United Kingdom, 4GCM Medical, San Juan, PR, 5CAICI SRL, Rosario, Argentina, 6Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China, 7AbbVie, Inc., North Chicago, IL
Background/Purpose: ABBV-599 is a novel combination of elsubrutinib (ELS; a selective BTK inhibitor) and upadacitinib (UPA; a JAK inhibitor) that targets non-overlapping signaling pathways associated with systemic lupus erythematosus (SLE). The objective of this analysis is to report results from SLEek, a phase 2, randomized, placebo (PBO)-controlled, parallel-group, multicenter study evaluating efficacy and safety of ABBV-599 and UPA monotherapy in adults with moderately to severely active SLE (NCT03978520).
Methods: Patients (pts) were randomized 1:1:1:1:1 to once daily (QD) ABBV-599 high dose (HD; ELS 60 mg + UPA 30 mg), ABBV-599 low dose (LD; ELS 60 mg + UPA 15 mg), ELS 60 mg, UPA 30 mg, or PBO. The primary endpoint was the proportion of patients at W24 achieving SLE Responder Index-4 (SRI-4) and steroid dose ≤ 10 mg QD; additional efficacy and safety endpoints through W48 are also reported. The pre-specified 2-sided alpha level was 0.1.
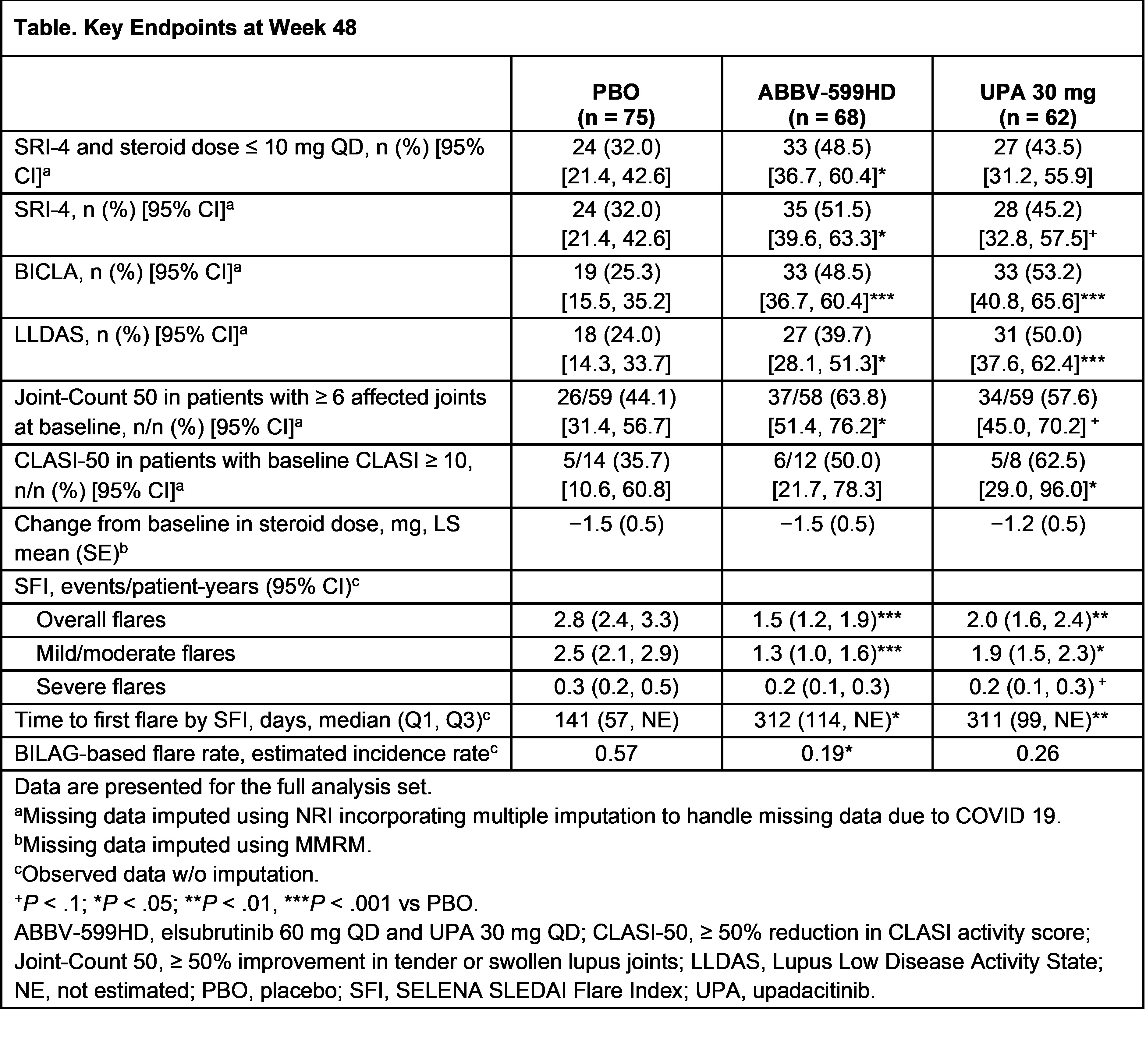
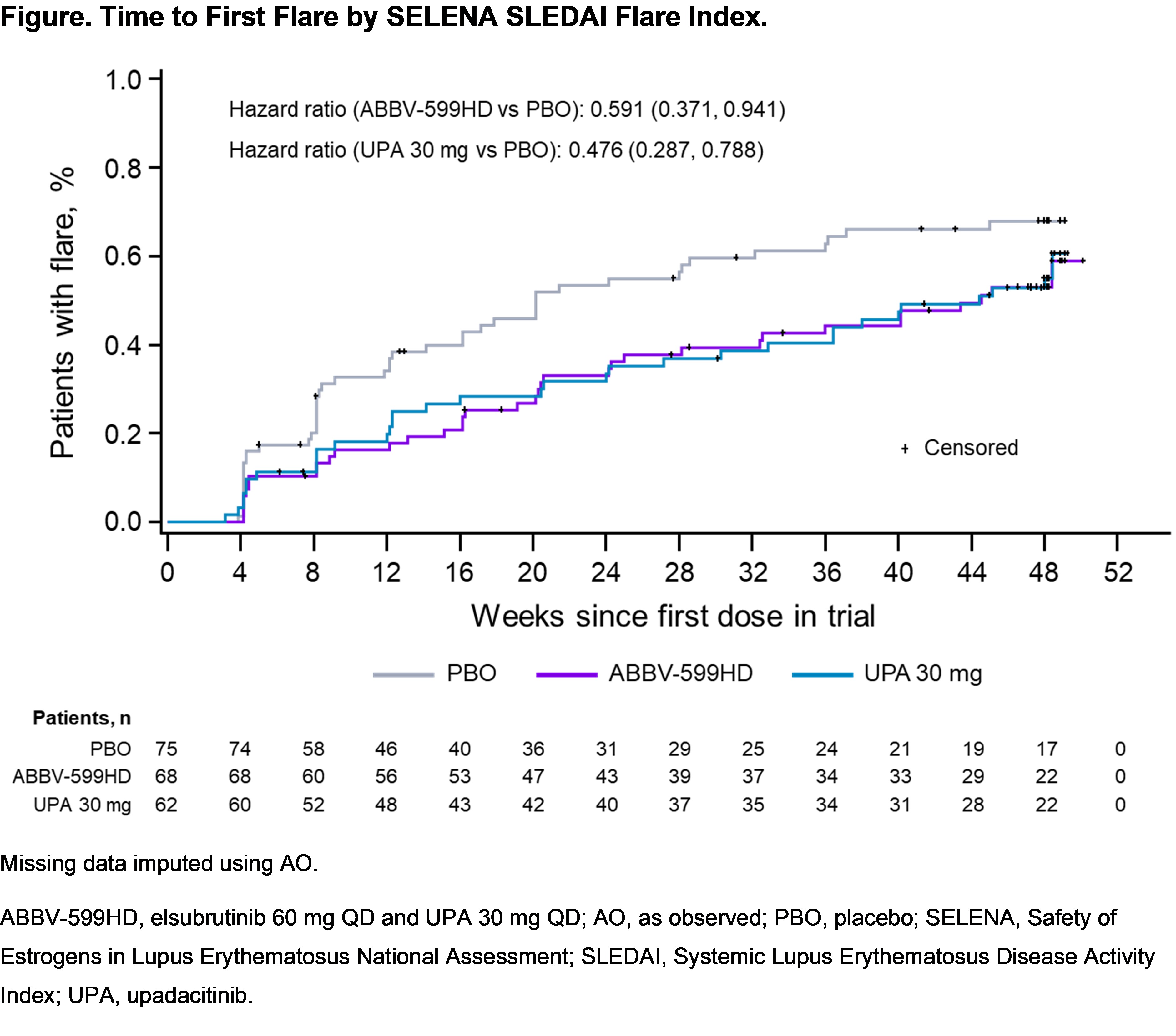
Results: 341 patients were enrolled. After a planned interim analysis when 50% of pts reached W24, the ABBV-599LD and ELS 60 mg arms were discontinued for lack of efficacy (no safety concerns). Of 205 continuing pts (ABBV-599HD n = 68, UPA 30 mg n = 62, PBO n = 75), baseline characteristics were well balanced. The primary endpoint (proportion achieving SRI-4 and steroid dose ≤ 10 mg QD at W24 vs PBO) was met by ABBV-599HD and UPA 30 mg. Key secondary endpoints were also achieved at W48 in both groups (Table). Overall flares and time to first flare were substantially reduced in the ABBV-599HD and UPA 30 mg groups through W48 (Figure). Anti-double stranded DNA antibodies were significantly decreased with both treatments. TEAEs considered related to study drug were 42.6% ABBV-599HD, 32.3% UPA 30 mg, and 33.3% PBO. There were no malignancies or VTE. There were 3 non-fatal CV events (1 MI on PBO and 2 ruptured cerebral aneurysms [1 each on ABBV-599HD and UPA 30 mg]); all were assessed as unrelated to study drug by investigators. No new safety signals were observed beyond previously known data for UPA or ELS.
Conclusion: ABBV-599HD (ELS 60 mg + UPA 30 mg) and UPA 30 mg demonstrated significant improvements in SLE disease activity and flares with acceptable safety through 48 weeks.
J. Merrill: AbbVie, 2, Alexion, 2, Alumis, 2, Amgen, 2, AstraZeneca, 2, 5, Aurinia, 2, Bristol Myers Squibb, 2, 5, EMD Serono, 2, Genentech, 2, Gilead, 2, GlaxoSmithKline, 2, 5, Lilly, 2, Merck, 2, Pfizer, 2, Provention, 2, Remegen, 2, Sanofi, 2, UCB Pharma, 2, Zenas, 2; Y. Tanaka: AbbVie, 6, AstraZeneca, 6, BMS, 6, Boehringer-Ingelheim, 6, Chugai, 5, 6, Eisai, 5, 6, Eli Lilly, 6, Gilead, 6, GSK, 6, Mitsubishi-Tanabe, 5, Pfizer, 6, Taiho, 6, Taisho, 5, 6; D. D'Cruz: Eli Lilly, 2, GlaxoSmithKlein(GSK), 2, UCB, 2; K. Vila-Rivera: AbbVie/Abbott, 2; D. Siri: AbbVie/Abbott, 5, Boehringer-Ingelheim, 5, Bristol-Myers Squibb(BMS), 5, Eli Lilly, 5, Gilead, 5, GlaxoSmithKlein(GSK), 5, Hoffman Laroche, 5, Janssen, 5, Sanofi, 5; X. Zeng: None; K. D'Silva: AbbVie/Abbott, 3, 11; L. Cheng: AbbVie/Abbott, 3, 11; T. Sornasse: AbbVie, 3, 11; T. Doan: AbbVie/Abbott, 3, 11; D. Kruzikas: AbbVie/Abbott, 3, 11; A. Friedman: AbbVie, 3, 11.
Background/Purpose: ABBV-599 is a novel combination of elsubrutinib (ELS; a selective BTK inhibitor) and upadacitinib (UPA; a JAK inhibitor) that targets non-overlapping signaling pathways associated with systemic lupus erythematosus (SLE). The objective of this analysis is to report results from SLEek, a phase 2, randomized, placebo (PBO)-controlled, parallel-group, multicenter study evaluating efficacy and safety of ABBV-599 and UPA monotherapy in adults with moderately to severely active SLE (NCT03978520).
Methods: Patients (pts) were randomized 1:1:1:1:1 to once daily (QD) ABBV-599 high dose (HD; ELS 60 mg + UPA 30 mg), ABBV-599 low dose (LD; ELS 60 mg + UPA 15 mg), ELS 60 mg, UPA 30 mg, or PBO. The primary endpoint was the proportion of patients at W24 achieving SLE Responder Index-4 (SRI-4) and steroid dose ≤ 10 mg QD; additional efficacy and safety endpoints through W48 are also reported. The pre-specified 2-sided alpha level was 0.1.
Results: 341 patients were enrolled. After a planned interim analysis when 50% of pts reached W24, the ABBV-599LD and ELS 60 mg arms were discontinued for lack of efficacy (no safety concerns). Of 205 continuing pts (ABBV-599HD n = 68, UPA 30 mg n = 62, PBO n = 75), baseline characteristics were well balanced. The primary endpoint (proportion achieving SRI-4 and steroid dose ≤ 10 mg QD at W24 vs PBO) was met by ABBV-599HD and UPA 30 mg. Key secondary endpoints were also achieved at W48 in both groups (Table). Overall flares and time to first flare were substantially reduced in the ABBV-599HD and UPA 30 mg groups through W48 (Figure). Anti-double stranded DNA antibodies were significantly decreased with both treatments. TEAEs considered related to study drug were 42.6% ABBV-599HD, 32.3% UPA 30 mg, and 33.3% PBO. There were no malignancies or VTE. There were 3 non-fatal CV events (1 MI on PBO and 2 ruptured cerebral aneurysms [1 each on ABBV-599HD and UPA 30 mg]); all were assessed as unrelated to study drug by investigators. No new safety signals were observed beyond previously known data for UPA or ELS.
Conclusion: ABBV-599HD (ELS 60 mg + UPA 30 mg) and UPA 30 mg demonstrated significant improvements in SLE disease activity and flares with acceptable safety through 48 weeks.


J. Merrill: AbbVie, 2, Alexion, 2, Alumis, 2, Amgen, 2, AstraZeneca, 2, 5, Aurinia, 2, Bristol Myers Squibb, 2, 5, EMD Serono, 2, Genentech, 2, Gilead, 2, GlaxoSmithKline, 2, 5, Lilly, 2, Merck, 2, Pfizer, 2, Provention, 2, Remegen, 2, Sanofi, 2, UCB Pharma, 2, Zenas, 2; Y. Tanaka: AbbVie, 6, AstraZeneca, 6, BMS, 6, Boehringer-Ingelheim, 6, Chugai, 5, 6, Eisai, 5, 6, Eli Lilly, 6, Gilead, 6, GSK, 6, Mitsubishi-Tanabe, 5, Pfizer, 6, Taiho, 6, Taisho, 5, 6; D. D'Cruz: Eli Lilly, 2, GlaxoSmithKlein(GSK), 2, UCB, 2; K. Vila-Rivera: AbbVie/Abbott, 2; D. Siri: AbbVie/Abbott, 5, Boehringer-Ingelheim, 5, Bristol-Myers Squibb(BMS), 5, Eli Lilly, 5, Gilead, 5, GlaxoSmithKlein(GSK), 5, Hoffman Laroche, 5, Janssen, 5, Sanofi, 5; X. Zeng: None; K. D'Silva: AbbVie/Abbott, 3, 11; L. Cheng: AbbVie/Abbott, 3, 11; T. Sornasse: AbbVie, 3, 11; T. Doan: AbbVie/Abbott, 3, 11; D. Kruzikas: AbbVie/Abbott, 3, 11; A. Friedman: AbbVie, 3, 11.



