Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Treatment II: Nonrenal (2485–2490)
2487: Phase 2 Safety and Efficacy of Subcutaneous (s.c.) Dose Ianalumab (VAY736; Anti-BAFFR mAb) Administered Monthly over 28 Weeks in Patients with Systemic Lupus Erythematosus (SLE) of Moderate-to-Severe Activity
Tuesday, November 14, 2023
2:30 PM - 2:40 PM PT
Location: Ballroom 20D
- NS
Nan Shen, MD, PhD
Shanghai Jiang Tong University School of Medicine
Shanghai, Shanghai, ChinaDisclosure(s): No financial relationships with ineligible companies to disclose
Presenting Author(s)
Nan Shen1, Stanislav Ignatenko2, Alexander Gordienko3, Josefina Cortés Hernández4, Nancy Agmon-Levin5, Pongthorn Narongroeknawin6, Katarzyna Romanowska -Prochnicka7, Hana Ciferska8, Masanari Kodera9, James Cheng-Chung Wei10, Piotr Leszczynski11, Joung-Liang Lan12, Eduardo Mysler13, Rafal Wojciechowski14, Tunde Tarr15, Elena Vishneva16, Yi-Hsing Chen17, Yuko Kaneko18, Stephanie Finzel19, Alberta Hoi20, Ajchara Koolvisoot21, Shin-Seok Lee22, Lie Dai23, Hiroshi Kaneko24, Bernadette Rojkovich25, Lingyun Sun26, Eugeny Zotkin27, Jean-Francois Viallard28, Masao Katayama29, Berta Paula Magallares-Lopez30, Tirtha Sengupta31, Carol Sips32 and Stephen J Oliver32, 1Shanghai Institute of Rheumatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University (SJTUSM), Shanghai, China, 2Charité Research Organisation GmbH, Berlin, Germany, 3SM Kirov Military Medical Academy, St. Petersburg, Russia, 4Lupus Unit, Rheumatology Department, Vall d’Hebron Hospitals, Barcelona, Spain, 5Yabludowicz Center for Autoimmune Disease, Sheba Medical Center, Tel Aviv University, Tel Aviv, Israel, 6Rheumatic Disease Unit, Department of Internal Medicine, Phramongkutklao Hospital and College of Medicine, Bangkok, Thailand, 7Department of Systemic Connective Tissue Diseases, National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland, 8Institute of Rheumatology and Department of Rheumatology, First Faculty of Medicine, Charles University, Prague, Czech Republic, 9Department of Dermatology, Japan Community Healthcare Organization Chukyo Hospital, Nagoya, Japan, 10Chung Shan Medical University Hospital, Department of Rheumatology, Taichung, Taiwan, 11Department of Internal Medicine, Poznan University of Medicine Sciences, Poznan, PL, Poznań, Poland, 12China Medical University Hospital, Taichung, Taiwan, 13Organizacion Medica de Investigacion, Buenos Aires, Argentina, 14Department of Rheumatology and Systemic Connective Tissue Diseases, University Hospital No. 2, Bydgoszcz, Poland, 15Division of Clinical Immunology, Faculty of Medicine, University of Debrecen, Debrecen, Hungary, 16LLC Family Clinic, Yekaterinburg, RU, Yekaterinburg, Russia, 17Taichung Veterans General Hospital, Taichung, Taiwan, 18Division of Rheumatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan, 19Department of Rheumatology and Clinical Immunology, University Medical Center Freiburg, University of Freiburg, Freiburg, Germany, 20Monash University, Department of Medicine, Sub-faculty of Clinical and Molecular Medicine, Melbourne, Australia, 21Division of Rheumatology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, 22Chonnam National University Medical School & Hospital, Gwangju, South Korea, 23Department of Rheumatology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China, 24Division of Rheumatic Disease, National Center for Global Health and Medicine, Tokyo, Japan, 25Department of Rheumatology and Physiotherapy, Polyclinic of the Hospitaller Brothers of St. John of God, Semmelweis University, Budapest, Hungary, 26Department of Rheumatology and Immunology, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China, 27VA Nasonova Research Institute of Rheumatology, Moscow, Russia, 28CHU de Bordeaux, Hôpital Haut-Lévêque, Pessac, France, 29National Hospital Organization, Nagoya Medical Center, Nagoya, JP, Nagoya, Japan, 30Department of Rheumatology, Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, 31Novartis Pharma India, Hyderabad, Hyderabad, India, 32Novartis Pharma AG, Basel, Switzerland
Background/Purpose: Ianalumab is a novel defucosylated human IgG1 mAb targeting the receptor for B cell Activating Factor belonging to the TNF Family (BAFF-R) providing potent B cell depletion by enhanced antibody-dependent cellular cytotoxicity along with BAFF:BAFF-R signaling blockade.We report safety and efficacy of ianalumab in patients with active SLE.
Methods: This multi-center randomized, parallel group, double-blind placebo-controlled umbrella trial (NCT03656562) enrolled patients (19 Dec 2018 to 31 Jan 2022) having ANA ≥1:80 and meeting ≥4 of 11 ACR 1997 SLE classification criteria, with SLEDAI-2K score ≥6 and BILAG-2004 ≥1 A or ≥2 B scores that included activity in either mucocutaneous and/or musculoskeletal domains. This report is limited to interim analysis results for the fully enrolled ianalumab treatment cohort (active n=34; placebo n=33) completing 28-week blinded treatment period (monthly s.c. injection ianalumab 300 mg or placebo), with measured outcomes at baseline and weeks (w) 4, 8, 12, 16, 24 & 28. Primary w28 outcome was proportion patients meeting composite endpoint requirements consisting of those achieving SLE-Responder Index (SRI)-4 who also tapered predniso(lo)ne to ≤5 mg/d or ≤ baseline dose, whichever was lower, by w16 and kept within that range to w28.Secondary/exploratory outcomes included safety/tolerability, incidence BILAG-2004 moderate or severe flares (≥1 A or ≥2 B), proportion patients achieving Lupus Low Disease Activity State (LLDAS), patient and physician global assessments, and laboratory markers of B cell-associated autoimmune activity.
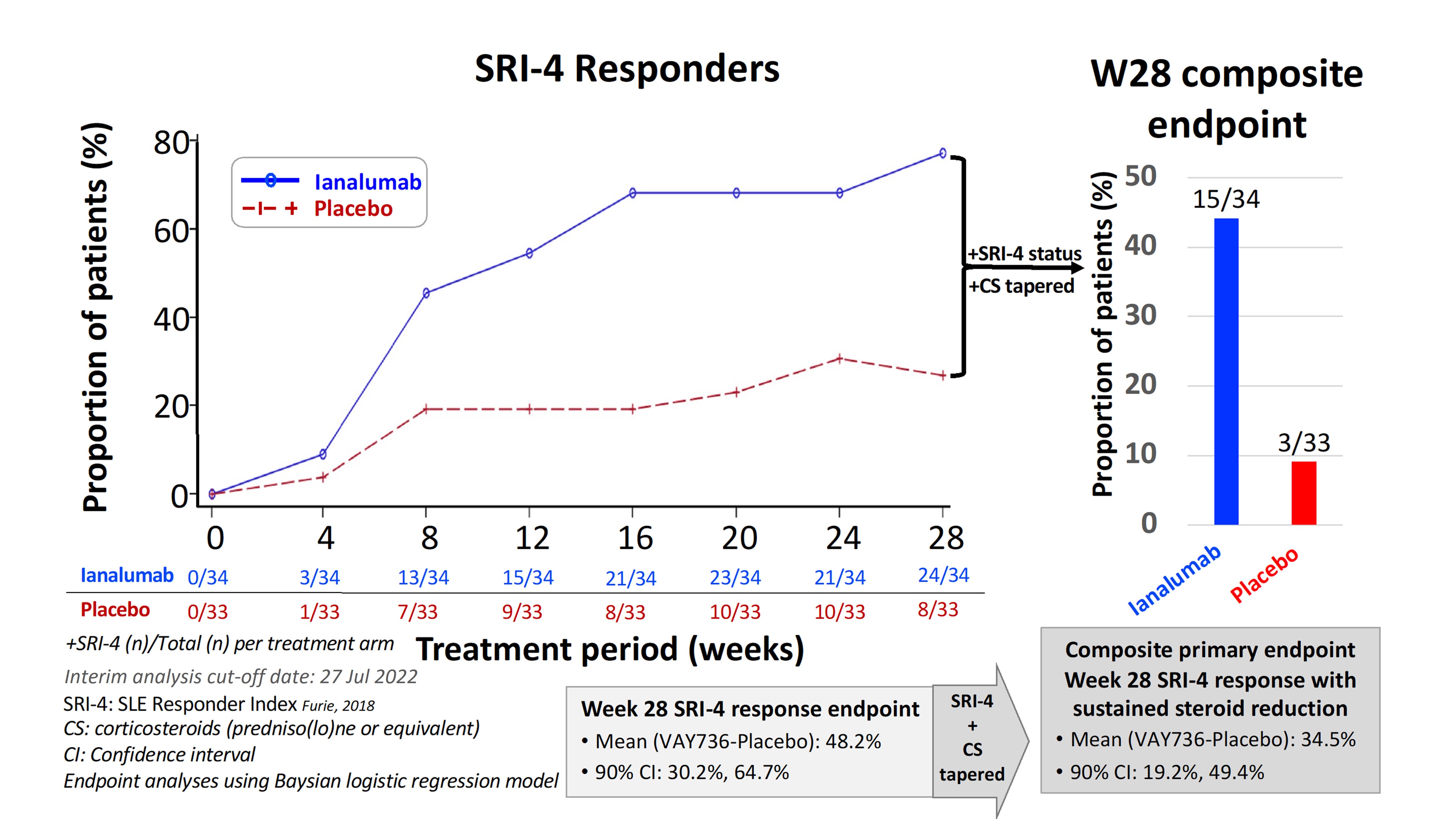
Results: VAY736 was safe and well-tolerated with no drug-related SAE or dropouts, and one pandemic-related discontinuation in placebo arm. Baseline median(range) values for ianalumab and placebo arms, respectively, were: SLEDAI-2Kscore 10 (6-32) and10 (4-18), and predniso(lo)ne 10 mg (0-30) and 10 mg (0-27.5). Marked depletion of circulating CD19+ B cells was consistently achieved in all ianalumab-treated patients: w28 median 1 cell/uL; range 0-3 (Fig. 3). The mean w28 SRI-4 treatment effect of ianalumab (n=24) over placebo (n=8) was 48.2% (Fig. 1), and that also met the composite primary endpoint was 34.5% (ianalumab n=15; placebo n=3). Reduced incidence of moderate or severe flare was noted for patients treated with ianalumab 44% (n=15) vs placebo 64% (n=21). Benefits of ianalumab over placebo were observed for time-to-moderate or -severe flare and for achieving w28 LLDAS (Fig. 2). Therapeutic responses were also observed for ianalumab- vs placebo-treated subjects on laboratory markers of autoimmune activity (Fig. 3).
Conclusion: Potent B cell depletion was consistently achieved in ianalumab-treated SLE patients that was well tolerated, achieving the primary endpoint of SRI-4 response with sustained steroid reduction, along with substantial treatment benefits on overall SRI-4 response, LLDAS and reductions in moderate and severe flares, and on laboratory markers of autoimmune activity. These positive phase 2 study results support ongoing phase 3 development in lupus of the dual mechanisms of action of ianalumab (SIRIUS-SLE 1 & 2, and SIRIUS-LN).
.jpg)
.jpg)
N. Shen: None; S. Ignatenko: None; A. Gordienko: None; J. Cortés Hernández: GSK, 6; N. Agmon-Levin: AstraZeneca, 1, 6, GlaxoSmithKlein(GSK), 1, 6, Novartis, 1, 6; P. Narongroeknawin: None; K. Romanowska -Prochnicka: None; H. Ciferska: None; M. Kodera: None; J. Wei: Abbvie, 2, 5, 6, Amgen, 5, AstraZeneca, 6, BMS, 2, 5, 6, Celgene, 2, Chugai, 2, 6, Eisai, 2, 6, Eli Lilly, 2, 5, 6, Gilead, 5, GSK, 2, 5, Janssen, 2, 5, 6, Novartis, 2, 5, Pfizer, 2, 5, 6, Sanofi-Aventis, 2, SUN pharma, 5, TSH Taiwan, 2, UCB pharma, 2, 5; P. Leszczynski: AbbVie/Abbott, 6, Eli Lilly, 6, Novartis, 6, Pfizer, 6, Roche, 5, UCB, 5; J. Lan: Annexon Biosciences, 5; E. Mysler: AbbVie, 1, 2, 6, Amgen, 6, AstraZeneca, 1, 5, 6, Bristol Myers Squibb, 5, GSK, 2, 5, Janssen, 1, 5, 6, Lilly, 5, 6, Novartis, 5, Pfizer, 1, 2, 6, Roche, 5, Sandoz, 6; R. Wojciechowski: Eli Lilly, 6, Novartis, 6; T. Tarr: None; E. Vishneva: None; Y. Chen: None; Y. Kaneko: AbbVie/Abbott, 1, 6, Ashai Kasei Pharma, 1, 6, Astellas Pharma, 1, 6, AstraZeneca, 1, 6, AYUMI Pharmacutia, 1, 6, Bristol-Myers Squibb(BMS), 1, 6, Chugai-Pharm, 1, 6, Eisai, 1, 6, Eli Lilly, 1, 6, Gilead Sciences Inc., 1, 6, GlaxoSmithKlein(GSK), 1, 6, Janssen Pharmaceutical KK, 1, 6, Novartis, 1, 6, Pfizer, 1, 6, Tanabe Mitsubishi Pharma, 1, 6, UCB Japan, 1, 6; S. Finzel: AbbVie, 6, AstraZeneca, 2, 6, Chugai, 6, Galapagos, 2, 6, Novartis, 2, Novo Nordisk, 2, UCB, 6; A. Hoi: Abbvie, 6, AstraZeneca, 5, Australian Rheumatology Association, 4, Eli Lilly, 6, EUSA Pharma (UK) Limited, 2, Limbic, 6, Moose Republic, 6, Novartis, 6; A. Koolvisoot: None; S. Lee: None; L. Dai: None; H. Kaneko: None; B. Rojkovich: None; L. Sun: None; E. Zotkin: None; J. Viallard: None; M. Katayama: None; B. Magallares-Lopez: None; T. Sengupta: Novartis, 3; C. Sips: Novartis, 3; S. J Oliver: Novartis, 3, 11.
Background/Purpose: Ianalumab is a novel defucosylated human IgG1 mAb targeting the receptor for B cell Activating Factor belonging to the TNF Family (BAFF-R) providing potent B cell depletion by enhanced antibody-dependent cellular cytotoxicity along with BAFF:BAFF-R signaling blockade.We report safety and efficacy of ianalumab in patients with active SLE.
Methods: This multi-center randomized, parallel group, double-blind placebo-controlled umbrella trial (NCT03656562) enrolled patients (19 Dec 2018 to 31 Jan 2022) having ANA ≥1:80 and meeting ≥4 of 11 ACR 1997 SLE classification criteria, with SLEDAI-2K score ≥6 and BILAG-2004 ≥1 A or ≥2 B scores that included activity in either mucocutaneous and/or musculoskeletal domains. This report is limited to interim analysis results for the fully enrolled ianalumab treatment cohort (active n=34; placebo n=33) completing 28-week blinded treatment period (monthly s.c. injection ianalumab 300 mg or placebo), with measured outcomes at baseline and weeks (w) 4, 8, 12, 16, 24 & 28. Primary w28 outcome was proportion patients meeting composite endpoint requirements consisting of those achieving SLE-Responder Index (SRI)-4 who also tapered predniso(lo)ne to ≤5 mg/d or ≤ baseline dose, whichever was lower, by w16 and kept within that range to w28.Secondary/exploratory outcomes included safety/tolerability, incidence BILAG-2004 moderate or severe flares (≥1 A or ≥2 B), proportion patients achieving Lupus Low Disease Activity State (LLDAS), patient and physician global assessments, and laboratory markers of B cell-associated autoimmune activity.
Results: VAY736 was safe and well-tolerated with no drug-related SAE or dropouts, and one pandemic-related discontinuation in placebo arm. Baseline median(range) values for ianalumab and placebo arms, respectively, were: SLEDAI-2Kscore 10 (6-32) and10 (4-18), and predniso(lo)ne 10 mg (0-30) and 10 mg (0-27.5). Marked depletion of circulating CD19+ B cells was consistently achieved in all ianalumab-treated patients: w28 median 1 cell/uL; range 0-3 (Fig. 3). The mean w28 SRI-4 treatment effect of ianalumab (n=24) over placebo (n=8) was 48.2% (Fig. 1), and that also met the composite primary endpoint was 34.5% (ianalumab n=15; placebo n=3). Reduced incidence of moderate or severe flare was noted for patients treated with ianalumab 44% (n=15) vs placebo 64% (n=21). Benefits of ianalumab over placebo were observed for time-to-moderate or -severe flare and for achieving w28 LLDAS (Fig. 2). Therapeutic responses were also observed for ianalumab- vs placebo-treated subjects on laboratory markers of autoimmune activity (Fig. 3).
Conclusion: Potent B cell depletion was consistently achieved in ianalumab-treated SLE patients that was well tolerated, achieving the primary endpoint of SRI-4 response with sustained steroid reduction, along with substantial treatment benefits on overall SRI-4 response, LLDAS and reductions in moderate and severe flares, and on laboratory markers of autoimmune activity. These positive phase 2 study results support ongoing phase 3 development in lupus of the dual mechanisms of action of ianalumab (SIRIUS-SLE 1 & 2, and SIRIUS-LN).

Figure 1. Primary endpoint: SRI-4 responders with sustained tapered corticosteroids
.jpg)
Figure 2. Ianalumab treatment effects on time to disease flare and achievement of LLDAS
.jpg)
Figure 3. Ianalumab effects on laboratory markers of B cell autoimmune activity
N. Shen: None; S. Ignatenko: None; A. Gordienko: None; J. Cortés Hernández: GSK, 6; N. Agmon-Levin: AstraZeneca, 1, 6, GlaxoSmithKlein(GSK), 1, 6, Novartis, 1, 6; P. Narongroeknawin: None; K. Romanowska -Prochnicka: None; H. Ciferska: None; M. Kodera: None; J. Wei: Abbvie, 2, 5, 6, Amgen, 5, AstraZeneca, 6, BMS, 2, 5, 6, Celgene, 2, Chugai, 2, 6, Eisai, 2, 6, Eli Lilly, 2, 5, 6, Gilead, 5, GSK, 2, 5, Janssen, 2, 5, 6, Novartis, 2, 5, Pfizer, 2, 5, 6, Sanofi-Aventis, 2, SUN pharma, 5, TSH Taiwan, 2, UCB pharma, 2, 5; P. Leszczynski: AbbVie/Abbott, 6, Eli Lilly, 6, Novartis, 6, Pfizer, 6, Roche, 5, UCB, 5; J. Lan: Annexon Biosciences, 5; E. Mysler: AbbVie, 1, 2, 6, Amgen, 6, AstraZeneca, 1, 5, 6, Bristol Myers Squibb, 5, GSK, 2, 5, Janssen, 1, 5, 6, Lilly, 5, 6, Novartis, 5, Pfizer, 1, 2, 6, Roche, 5, Sandoz, 6; R. Wojciechowski: Eli Lilly, 6, Novartis, 6; T. Tarr: None; E. Vishneva: None; Y. Chen: None; Y. Kaneko: AbbVie/Abbott, 1, 6, Ashai Kasei Pharma, 1, 6, Astellas Pharma, 1, 6, AstraZeneca, 1, 6, AYUMI Pharmacutia, 1, 6, Bristol-Myers Squibb(BMS), 1, 6, Chugai-Pharm, 1, 6, Eisai, 1, 6, Eli Lilly, 1, 6, Gilead Sciences Inc., 1, 6, GlaxoSmithKlein(GSK), 1, 6, Janssen Pharmaceutical KK, 1, 6, Novartis, 1, 6, Pfizer, 1, 6, Tanabe Mitsubishi Pharma, 1, 6, UCB Japan, 1, 6; S. Finzel: AbbVie, 6, AstraZeneca, 2, 6, Chugai, 6, Galapagos, 2, 6, Novartis, 2, Novo Nordisk, 2, UCB, 6; A. Hoi: Abbvie, 6, AstraZeneca, 5, Australian Rheumatology Association, 4, Eli Lilly, 6, EUSA Pharma (UK) Limited, 2, Limbic, 6, Moose Republic, 6, Novartis, 6; A. Koolvisoot: None; S. Lee: None; L. Dai: None; H. Kaneko: None; B. Rojkovich: None; L. Sun: None; E. Zotkin: None; J. Viallard: None; M. Katayama: None; B. Magallares-Lopez: None; T. Sengupta: Novartis, 3; C. Sips: Novartis, 3; S. J Oliver: Novartis, 3, 11.



