Poster Session A
Crystal arthropathies
Session: (0229–0251) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
0231: Febuxostat Dose Requirement for Achieving Target Serum Urate Levels According to Renal Function: A Retrospective Cohort Study
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- HS
Hansun Song, MD (he/him/his)
Asan medical center
Seoul, South KoreaDisclosure information not submitted.
Abstract Poster Presenter(s)
YoungEun Kim1, Hansun Song1, Wook Jang Seo2, Jinseok Kim3, Soo Min Ahn1, Ji-Seon Oh4, Yong-Gil Kim1, Chang-Keun Lee5, Bin Yoo5 and Seokchan Hong6, 1Department of Rheumatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea, 2Department of Rheumatology, Veteran Health Service Medical Center, Seoul, South Korea, 3Department of Rheumatology, Jeju National University Hospital, Jeju National University School of Medicine, Jeju-si, Jeju-do, South Korea, La Jolla, CA, South Korea, 4Department of Information Medicine, Big Data Research Center, Asan Medical Center, University of Ulsan College of Medicine, Ulsan, South Korea, 5Asan Medical Center, Seoul, South Korea, 6Department of Rheumatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
Background/Purpose: Febuxostat clearance is not affected by kidney function, and the risk of adverse events from allopurinol, including fatal hypersensitivity reactions, is higher in patients with renal dysfunction accordingly, febuxostat is preferred for gout patients with chronic kidney disease (CKD). However, few studies have sought to determine the appropriate febuxostat dose required to achieve the target SU level. Therefore, this study aimed to investigate the febuxostat dosage needed to reach the SU target in patients with gouty arthritis, including those with renal impairment.
Methods: We conducted a retrospective cohort study at Asan Medical Center, a tertiary referral hospital in Seoul, South Korea. Of 3153 gout patients who were prescribed febuxostat, 731 patients with an initial SU > 6 mg/dL were included and categorized into three groups based on their estimated glomerular filtration rate (eGFR): chronic kidney disease (CKD) stage 1, stages 2-3, and stages 4-5. The cumulative febuxostat dose was defined as the total dose administered from initiation until the patient reached the target SU ( < 6 mg/dL).
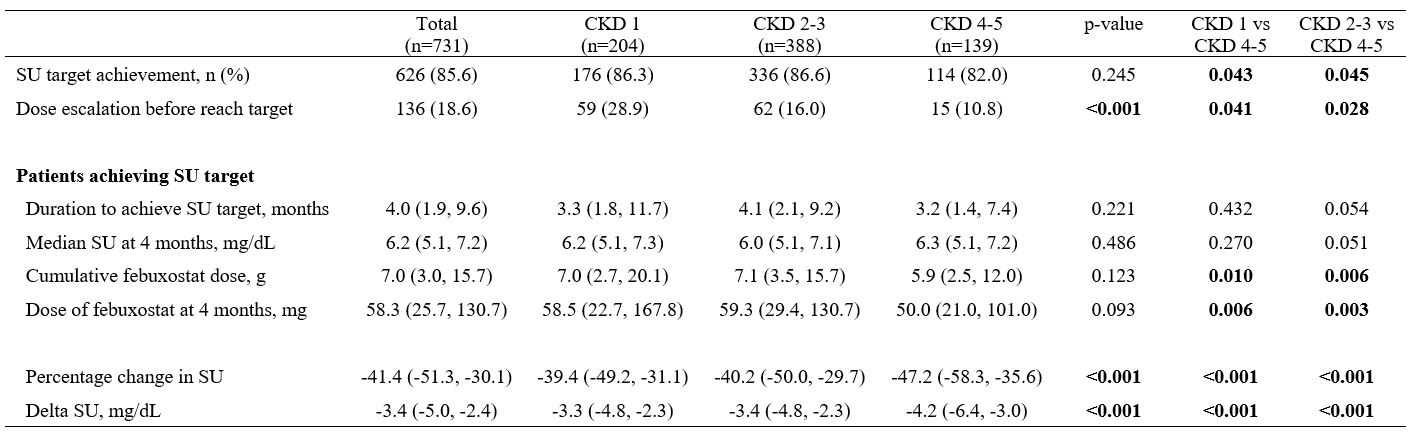
Results: The cohort of 731 gout patients had a median age of 52 years (IQR, 41-63) and comprised 667 (91.2%) men. The mean (± standard deviation) SU at febuxostat initiation was significantly higher in the CKD 4-5 group (9.6 [±3.1] mg/dL) than in the other groups (CKD 2-3 group, 8.6 [±1.6]; CKD 1 group, 8.8 [±1.8]; P < 0.001). The proportion of patients who received an initial febuxostat dose of 80 mg was lower in the CKD 2-3 group (30.7%) and CKD 4-5 group (31.7%) than in the CKD 1 group (52.0%). Of the total patients, 626 (85.6%) achieved the SU target after ULT. The proportions of patients who achieved the target SU levels were lower in the CKD 4-5 group (82.0%) than in the CKD 1 group (86.6%) or CKD 2-3 group (86.3%) (P = 0.045). Notably, the cumulative febuxostat dose was significantly lower in the CKD 4-5 group (5.9 g [IQR, 2.5–12.0]) than in the other groups (CKD 2-3, 7.1 g [IQR, 3.5–15.7], P = 0.006; CKD 1, 7.0 g [IQR, 2.7–20.1]; P = 0.010). Furthermore, the CKD 4-5 group had a significant negative correlation with the cumulative febuxostat dosage required to reach the target SU compared to the CKD 1 group (beta -2.334, P = 0.020).
Conclusion: Patients with severely decreased renal function (CKD 4-5) required a significantly lower febuxostat dose to achieve the target SU level.
Y. Kim: None; H. Song: None; W. Seo: None; J. Kim: None; S. Ahn: None; J. Oh: None; Y. Kim: None; C. Lee: None; B. Yoo: None; S. Hong: None.
Background/Purpose: Febuxostat clearance is not affected by kidney function, and the risk of adverse events from allopurinol, including fatal hypersensitivity reactions, is higher in patients with renal dysfunction accordingly, febuxostat is preferred for gout patients with chronic kidney disease (CKD). However, few studies have sought to determine the appropriate febuxostat dose required to achieve the target SU level. Therefore, this study aimed to investigate the febuxostat dosage needed to reach the SU target in patients with gouty arthritis, including those with renal impairment.
Methods: We conducted a retrospective cohort study at Asan Medical Center, a tertiary referral hospital in Seoul, South Korea. Of 3153 gout patients who were prescribed febuxostat, 731 patients with an initial SU > 6 mg/dL were included and categorized into three groups based on their estimated glomerular filtration rate (eGFR): chronic kidney disease (CKD) stage 1, stages 2-3, and stages 4-5. The cumulative febuxostat dose was defined as the total dose administered from initiation until the patient reached the target SU ( < 6 mg/dL).
Results: The cohort of 731 gout patients had a median age of 52 years (IQR, 41-63) and comprised 667 (91.2%) men. The mean (± standard deviation) SU at febuxostat initiation was significantly higher in the CKD 4-5 group (9.6 [±3.1] mg/dL) than in the other groups (CKD 2-3 group, 8.6 [±1.6]; CKD 1 group, 8.8 [±1.8]; P < 0.001). The proportion of patients who received an initial febuxostat dose of 80 mg was lower in the CKD 2-3 group (30.7%) and CKD 4-5 group (31.7%) than in the CKD 1 group (52.0%). Of the total patients, 626 (85.6%) achieved the SU target after ULT. The proportions of patients who achieved the target SU levels were lower in the CKD 4-5 group (82.0%) than in the CKD 1 group (86.6%) or CKD 2-3 group (86.3%) (P = 0.045). Notably, the cumulative febuxostat dose was significantly lower in the CKD 4-5 group (5.9 g [IQR, 2.5–12.0]) than in the other groups (CKD 2-3, 7.1 g [IQR, 3.5–15.7], P = 0.006; CKD 1, 7.0 g [IQR, 2.7–20.1]; P = 0.010). Furthermore, the CKD 4-5 group had a significant negative correlation with the cumulative febuxostat dosage required to reach the target SU compared to the CKD 1 group (beta -2.334, P = 0.020).
Conclusion: Patients with severely decreased renal function (CKD 4-5) required a significantly lower febuxostat dose to achieve the target SU level.

Febuxostat acquirement to reach target SU level and comparison between patients with CKD 1, 2-3, and 4-5.
Y. Kim: None; H. Song: None; W. Seo: None; J. Kim: None; S. Ahn: None; J. Oh: None; Y. Kim: None; C. Lee: None; B. Yoo: None; S. Hong: None.



