Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Etiology & Pathogenesis (2431–2436)
2433: Longitudinal Multi-Omics Single Cell Analysis Reveals Abatacept Treatment Shifts Peripheral Lymphocyte Subpopulations in Seropositive RA with Reduction of Mature B Cells and Retention of Transitional and Naive B Cells
Tuesday, November 14, 2023
2:30 PM - 2:40 PM PT
Location: Room 29A-D
- JS
Jasmine Shwetar
New York School of Medicine
Ann Arbor, MI, United StatesDisclosure information not submitted.
Presenting Author(s)
Gregg Silverman1, William Rigby2, Helena Jun1, Jasmine Shwetar1, Katie Tumang1, Sergei Koralov3, Ellie Ivanova1, David Mieles1, Sladjana Skopelia-Gardner4 and Kelly Ruggles1, 1NYU Grossman School of Medicine, New York, NY, 2Hitchcock-Dartmouth Medicine Center, Hanover, NH, 3NYU Grossman Schoolof Medicine, New York, NY, 4Hitchcock-Dartmouth Medicine Center, Lebanon, NH
Background/Purpose: Biologic agents of diverse molecular mechanisms of action are approved for RA, but we do not have a full understanding of the implications of treatment and we do not know who will best respond to an agent. Abatacept (CTLA4-Ig) was designed to block a co-stimulation pathway involved in T-cell activation, yet other effects have been suspected. We therefore designed the open-label study, RA and memory B cells (RAMBA trial), to investigate the cellular and transcriptomic consequences of abatacept treatment.
Methods: Biologic-naïve patients with seropositive RA were enrolled, and received the standard IV regimen of abatacept that was then discontinued after the month 5 infusion. Clinical evaluation and biospecimen collection were performed at baseline, 3, 6, and 9 months or when a flare occurred. In addition to routine clinical lab testing, autoantibody responses were evaluated in a multiplex assay with IgM-RF, and IgM- and IgG- to 4 ACPA fine specificities including CCP3. PBMCs or samples of negatively selected B-lineage cells were interrogated at a single cell level for high-dimensional surface phenotype and transcriptomic profiles, by Expanded Cellular Indexing of Transcriptomes and Epitopes (ExCITE) sequencing.
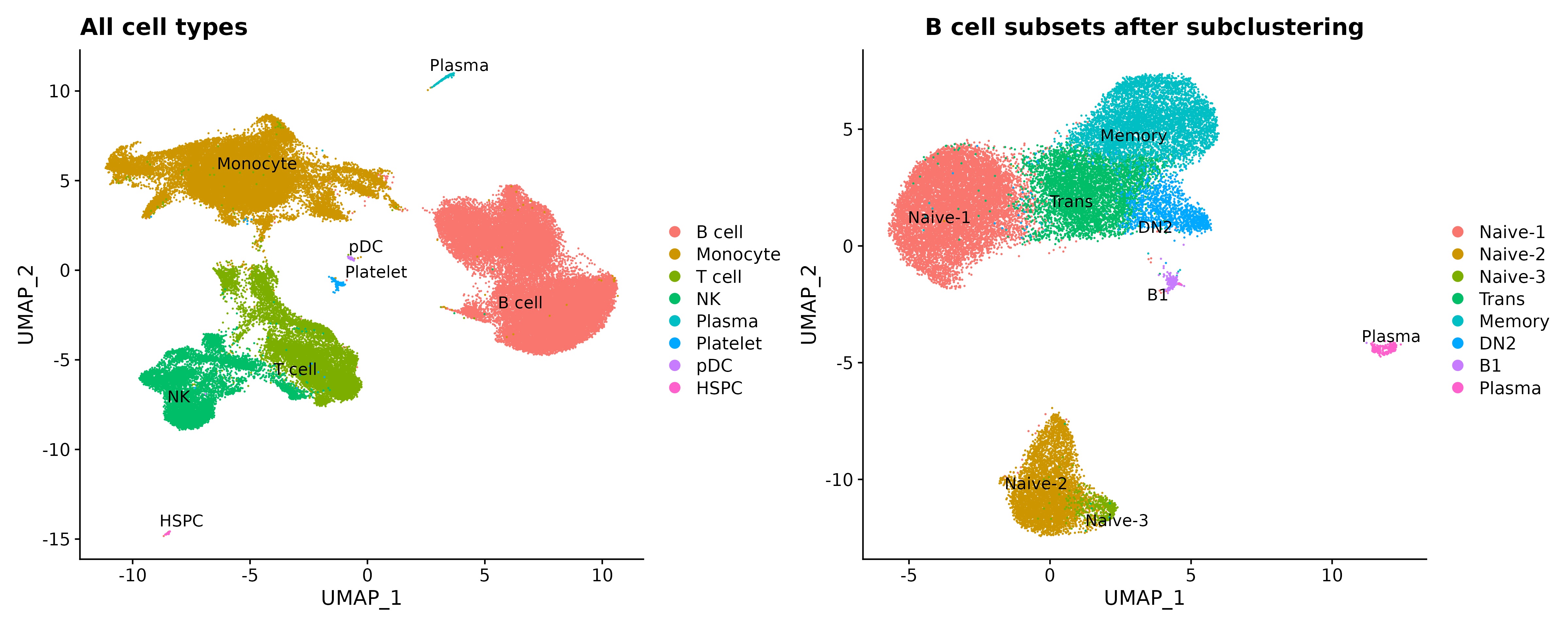
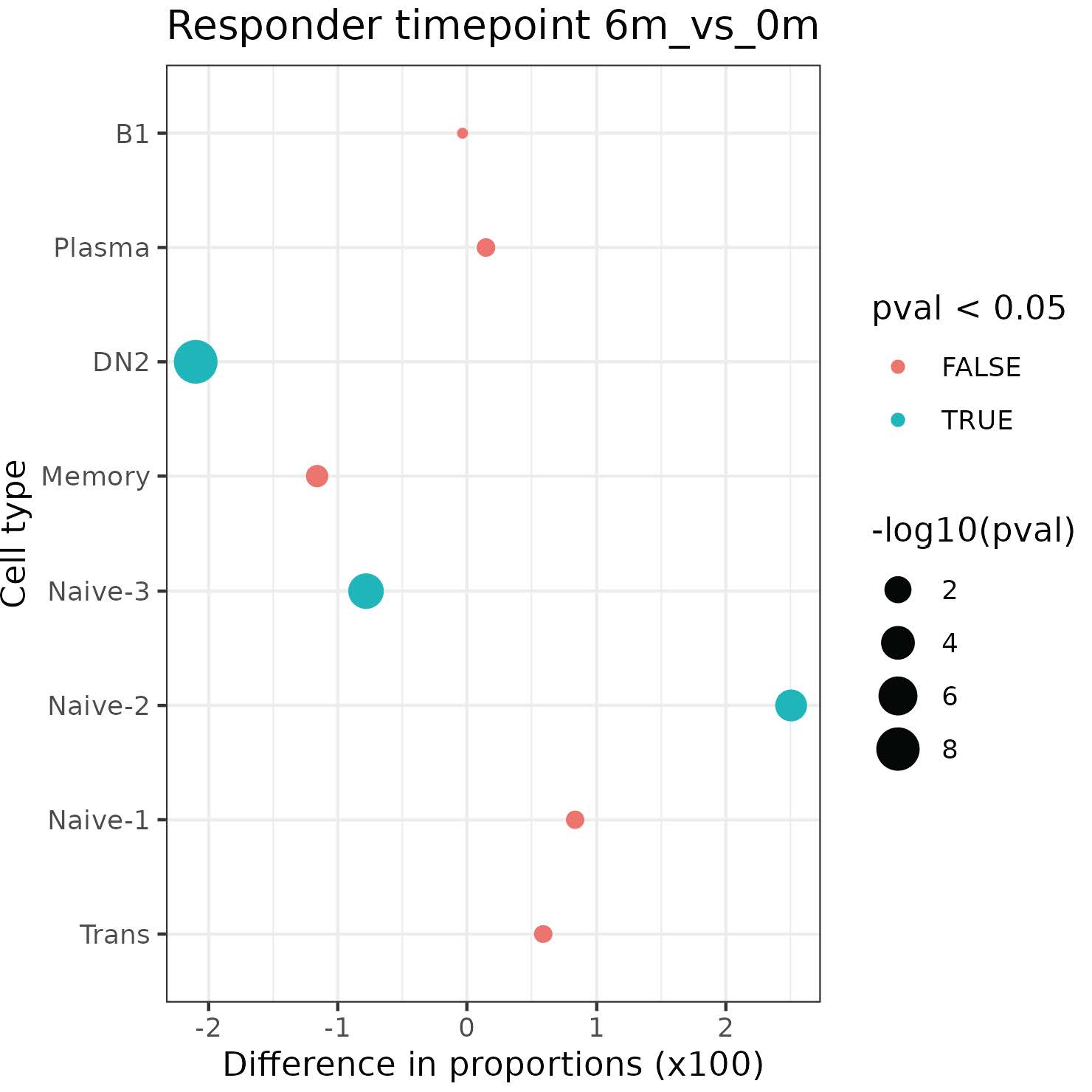
Results: 21 enrolled patients had two or more follow-up visits, with 18 completing to 9 months or post-treatment flare.12 patients achieved low disease activity by DAS28-CRP (< 3.2) or CDAI (< 10). Clinical responses were mirrored by reductions in serum RF and/or ACPA levels (not shown). Initial single cell analyses of 7 patients, of 70,790 PBMCs and 33,160 B-lineage cells Abatacept treatments induced increases in naïve CD4+ T cells over time (not shown). CD19+ B-lineage cells were resolved into 8 subsets (Fig.1). Treatment was associated with significant reductions of Naïve-3, and CD19+ sIgD- CD27- (DN2) B cells linked to autoimmune B-cell responses. In contrast, treatment was also associated with significant increases in the accumulation of Naïve-2 B cells, and a trend towards increases in the peripheral accumulation of transitional and Naïve-1 B cells (Fig.2).
Conclusion: Complete clinical response to abatacept in RA was associated with cellular shifts in peripheral B-cell subsets identified based on surface phenotype and transcriptomic profiles, with a return to a state dominated by newly emergent transitional and early naïve B cells. These findings elucidate previously unsuspected aspects of MOA of abatacept, that we postulate reflects a reversion in balance towards the preimmune natural state of clonal ignorance unaffected by autoimmune disease.
Supported in part by funds from BMS.
G. Silverman: None; W. Rigby: None; H. Jun: None; J. Shwetar: None; K. Tumang: None; S. Koralov: None; E. Ivanova: None; D. Mieles: None; S. Skopelia-Gardner: None; K. Ruggles: None.
Background/Purpose: Biologic agents of diverse molecular mechanisms of action are approved for RA, but we do not have a full understanding of the implications of treatment and we do not know who will best respond to an agent. Abatacept (CTLA4-Ig) was designed to block a co-stimulation pathway involved in T-cell activation, yet other effects have been suspected. We therefore designed the open-label study, RA and memory B cells (RAMBA trial), to investigate the cellular and transcriptomic consequences of abatacept treatment.
Methods: Biologic-naïve patients with seropositive RA were enrolled, and received the standard IV regimen of abatacept that was then discontinued after the month 5 infusion. Clinical evaluation and biospecimen collection were performed at baseline, 3, 6, and 9 months or when a flare occurred. In addition to routine clinical lab testing, autoantibody responses were evaluated in a multiplex assay with IgM-RF, and IgM- and IgG- to 4 ACPA fine specificities including CCP3. PBMCs or samples of negatively selected B-lineage cells were interrogated at a single cell level for high-dimensional surface phenotype and transcriptomic profiles, by Expanded Cellular Indexing of Transcriptomes and Epitopes (ExCITE) sequencing.
Results: 21 enrolled patients had two or more follow-up visits, with 18 completing to 9 months or post-treatment flare.12 patients achieved low disease activity by DAS28-CRP (< 3.2) or CDAI (< 10). Clinical responses were mirrored by reductions in serum RF and/or ACPA levels (not shown). Initial single cell analyses of 7 patients, of 70,790 PBMCs and 33,160 B-lineage cells Abatacept treatments induced increases in naïve CD4+ T cells over time (not shown). CD19+ B-lineage cells were resolved into 8 subsets (Fig.1). Treatment was associated with significant reductions of Naïve-3, and CD19+ sIgD- CD27- (DN2) B cells linked to autoimmune B-cell responses. In contrast, treatment was also associated with significant increases in the accumulation of Naïve-2 B cells, and a trend towards increases in the peripheral accumulation of transitional and Naïve-1 B cells (Fig.2).
Conclusion: Complete clinical response to abatacept in RA was associated with cellular shifts in peripheral B-cell subsets identified based on surface phenotype and transcriptomic profiles, with a return to a state dominated by newly emergent transitional and early naïve B cells. These findings elucidate previously unsuspected aspects of MOA of abatacept, that we postulate reflects a reversion in balance towards the preimmune natural state of clonal ignorance unaffected by autoimmune disease.
Supported in part by funds from BMS.

Figure 1. Cell type clustering based on UMAP analysis for 7 RA patients. Data pooled from samples obtained at baseline (0month), and 3 months and 6 months after abatacept initiation, with discontinuation after month 5 infusion, and follow-up at 9 months.

Figure 2. Distribution of B-cell subsets in clinical responders over time. Results are for Chi-squared test on the counts of responder B cell subsets between baseline and 6 month time points.
G. Silverman: None; W. Rigby: None; H. Jun: None; J. Shwetar: None; K. Tumang: None; S. Koralov: None; E. Ivanova: None; D. Mieles: None; S. Skopelia-Gardner: None; K. Ruggles: None.



