Abstract Session
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: Abstracts: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science I (2461–2466)
2461: Transcriptional Derepression of CHD4/NuRD-regulated Genes in the Muscle of Patients with Dermatomyositis and anti-Mi2 Autoantibodies
Tuesday, November 14, 2023
2:00 PM - 2:10 PM PT
Location: Room 25A-C
- IP
Iago Pinal-Fernandez, MD, PhD
National Institutes of Health
Bethesda, MD, United StatesDisclosure information not submitted.
Presenting Author(s)
Iago Pinal-Fernandez1, Jose Cesar Milisenda2, Katherine Pak1, Sandra Muñoz-Braceras3, Maria Casal-Dominguez3, Jose Jiram Torres-Ruiz3, Stefania Dell´Orso4, Faiza Naz4, Gustavo Gutierrez-Cruz4, Yaiza Duque-Jaimez2, Ana Matas-Garcia2, Joan Padrosa5, Francesc J Garcia-Garcia2, Mariona Guitart-Manpel2, Gloria Garrabou2, Ernesto Trallero-Araguas6, Brian Wallit7, Julie Paik8, Jemima Albayda8, Lisa Christopher-Stine8, Tom Lloyd9, Josep Maria Grau2, Albert Selva-O’Callaghan6 and Andrew Mammen10, 1National Institutes of Health, Bethesda, MD, 2Muscle Research Unit, Internal Medicine Service, Hospital Clinic, Barcelona, Spain, 3Muscle Disease Unit, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, 4National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, 5CIBERER, Barcelona, Spain, Barcelona, Spain, 6Systemic Autoimmune Disease Unit, Vall d’Hebron Institute of Research, Barcelona, Spain, 7National Institute of Neurological Diseases and Stroke, National Institutes of Health, Bethesda, MD, 8Johns Hopkins University, Baltimore, MD, 9Johns Hopkins University School of Medicine, Baltimore, MD, 10NIH, Bethesda, MD
Background/Purpose: Myositis is a heterogeneous family of diseases including dermatomyositis (DM), immune-mediated necrotizing myopathy (IMNM), antisynthetase syndrome (AS), and inclusion body myositis (IBM). Myositis-specific autoantibodies define different subtypes of myositis. For example, patients with anti-Mi2 autoantibodies targeting the CHD4/NuRD complex (a transcriptional repressor) have more severe muscle disease than other DM patients. This study aimed to define the transcriptional profile of muscle biopsies from anti-Mi2-positive DM patients.
Methods: RNA sequencing was performed on muscle biopsies (n=171) from patients with anti-Mi2-positive DM (n=18), DM without anti-Mi2 autoantibodies (n=32), AS (n=18), IMNM (n=54), and IBM (n=16) as well as 33 normal muscle biopsies. Genes specifically upregulated in anti-Mi2-positive DM were identified. Muscle biopsies were stained for human immunoglobulin and protein products corresponding to genes specifically upregulated in anti-Mi2-positive muscle biopsies.
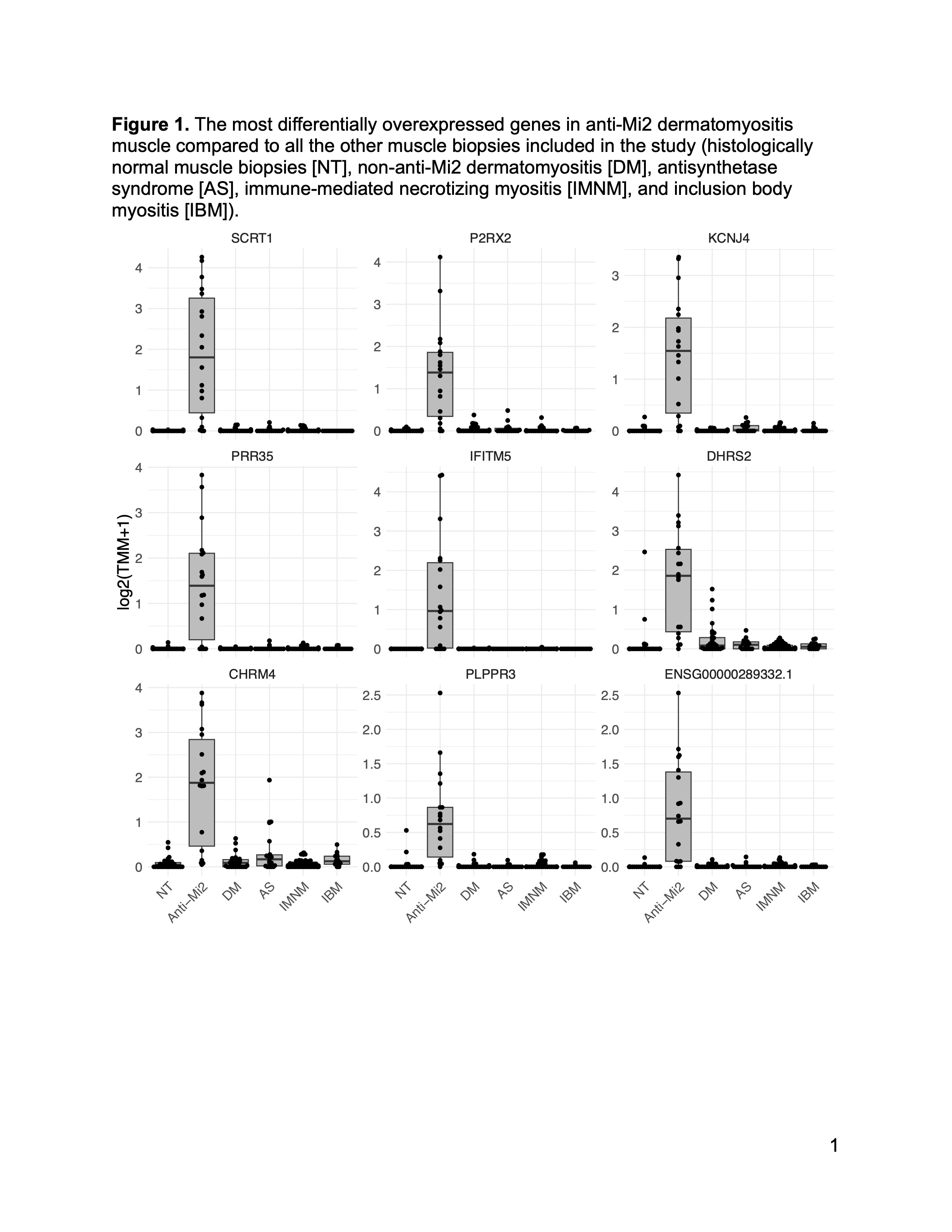
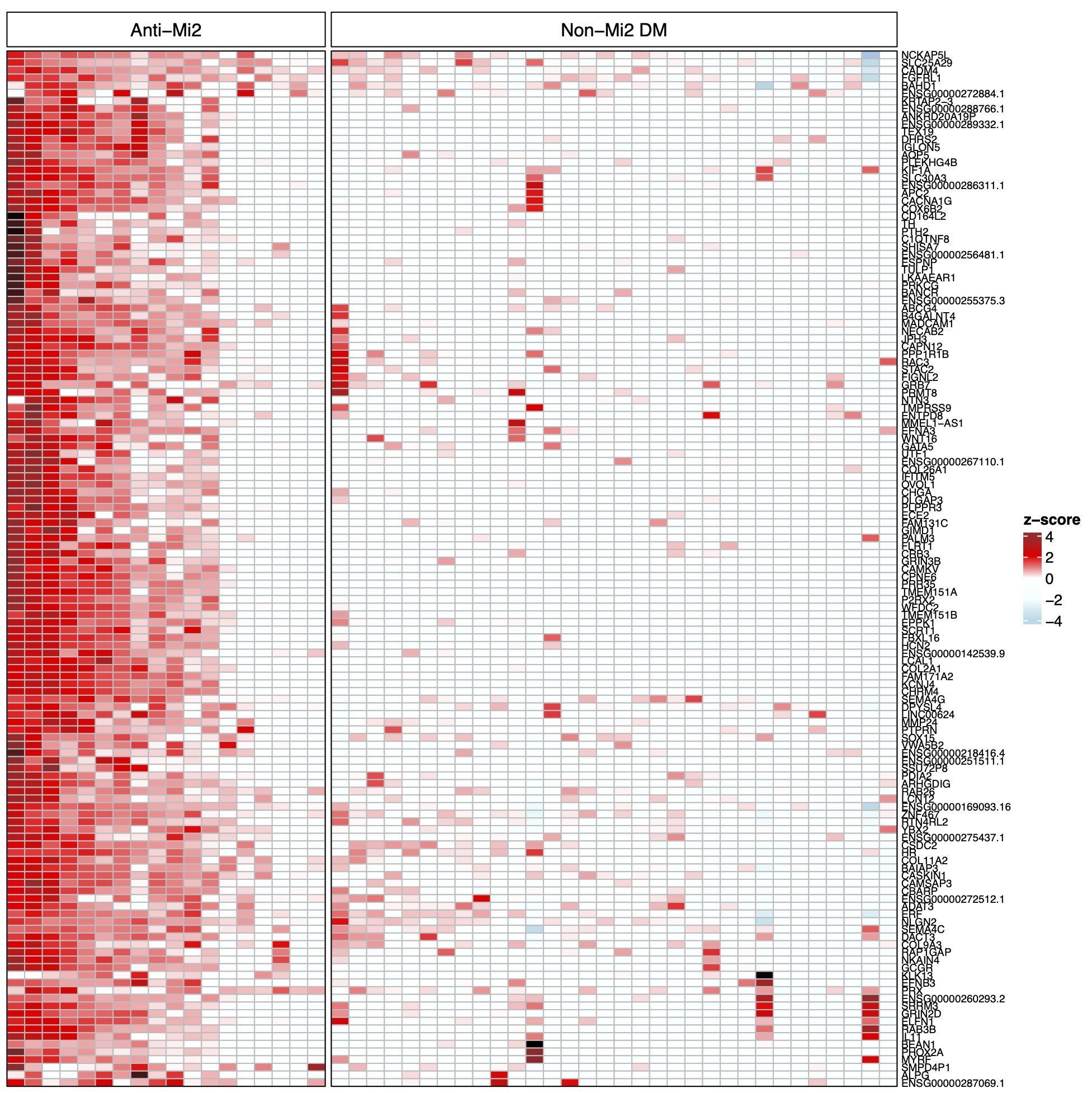
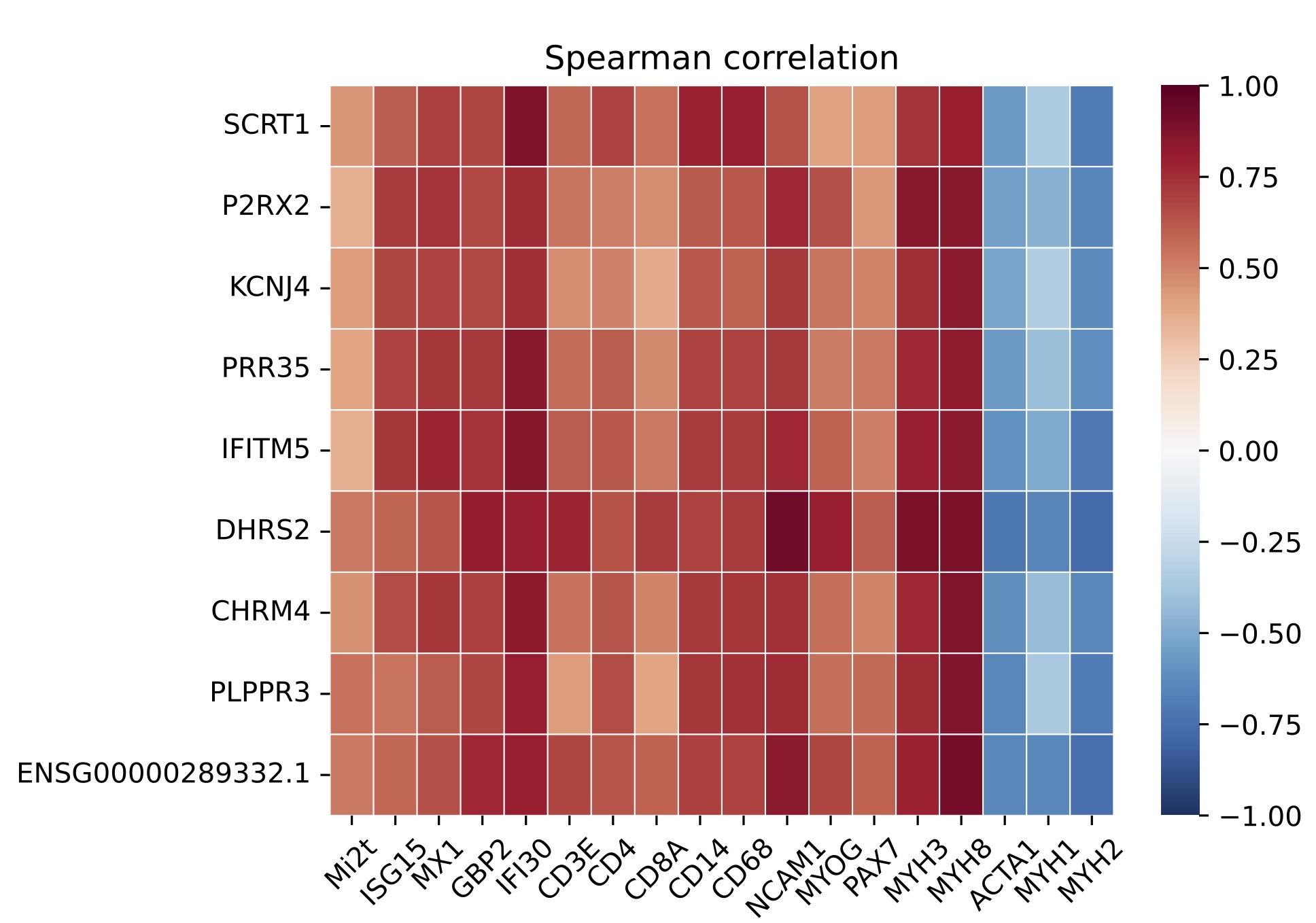
Results: A set of 135 genes, including SCRT1 and MADCAM, was specifically overexpressed in anti-Mi2-positive DM muscle. This set was enriched for CHD4/NuRD-regulated genes and included genes that are not otherwise expressed in skeletal muscle. The expression levels of these genes correlated with anti-Mi2 autoantibody titers, markers of disease activity, and with the other members of the gene set. In anti-Mi2-positive muscle biopsies, immunoglobulin was localized to the nucleus, MADCAM protein was present in the cytoplasm of perifascicular fibers, and SCRT1 protein was localized to myofiber nuclei.
Conclusion: Based on these findings, we hypothesize that anti-Mi2 autoantibodies could exert a pathogenic effect by entering damaged myofibers, inhibiting the CHD4/NuRD complex, and subsequently derepressing the unique set of genes defined in this study.
I. Pinal-Fernandez: None; J. Milisenda: None; K. Pak: None; S. Muñoz-Braceras: None; M. Casal-Dominguez: None; J. Torres-Ruiz: None; S. Dell´Orso: None; F. Naz: None; G. Gutierrez-Cruz: None; Y. Duque-Jaimez: None; A. Matas-Garcia: None; J. Padrosa: None; F. Garcia-Garcia: None; M. Guitart-Manpel: None; G. Garrabou: None; E. Trallero-Araguas: None; B. Wallit: None; J. Paik: None; J. Albayda: Amgen, 5, Janssen, 5; L. Christopher-Stine: None; T. Lloyd: None; J. Grau: None; A. Selva-O’Callaghan: None; A. Mammen: None.
Background/Purpose: Myositis is a heterogeneous family of diseases including dermatomyositis (DM), immune-mediated necrotizing myopathy (IMNM), antisynthetase syndrome (AS), and inclusion body myositis (IBM). Myositis-specific autoantibodies define different subtypes of myositis. For example, patients with anti-Mi2 autoantibodies targeting the CHD4/NuRD complex (a transcriptional repressor) have more severe muscle disease than other DM patients. This study aimed to define the transcriptional profile of muscle biopsies from anti-Mi2-positive DM patients.
Methods: RNA sequencing was performed on muscle biopsies (n=171) from patients with anti-Mi2-positive DM (n=18), DM without anti-Mi2 autoantibodies (n=32), AS (n=18), IMNM (n=54), and IBM (n=16) as well as 33 normal muscle biopsies. Genes specifically upregulated in anti-Mi2-positive DM were identified. Muscle biopsies were stained for human immunoglobulin and protein products corresponding to genes specifically upregulated in anti-Mi2-positive muscle biopsies.
Results: A set of 135 genes, including SCRT1 and MADCAM, was specifically overexpressed in anti-Mi2-positive DM muscle. This set was enriched for CHD4/NuRD-regulated genes and included genes that are not otherwise expressed in skeletal muscle. The expression levels of these genes correlated with anti-Mi2 autoantibody titers, markers of disease activity, and with the other members of the gene set. In anti-Mi2-positive muscle biopsies, immunoglobulin was localized to the nucleus, MADCAM protein was present in the cytoplasm of perifascicular fibers, and SCRT1 protein was localized to myofiber nuclei.
Conclusion: Based on these findings, we hypothesize that anti-Mi2 autoantibodies could exert a pathogenic effect by entering damaged myofibers, inhibiting the CHD4/NuRD complex, and subsequently derepressing the unique set of genes defined in this study.

Figure 1. The most differentially overexpressed genes in anti-Mi2 dermatomyositis muscle compared to all the other muscle biopsies included in the study (histologically normal muscle biopsies [NT], non-anti-Mi2 dermatomyositis [DM], antisynthetase syndrome [AS], immune-mediated necrotizing myositis [IMNM], and inclusion body myositis [IBM]).

Figure 2. Normalized expression (z-score) of the specifically overexpressed genes in patients with anti-Mi2-positive dermatomyositis (DM) compared with non-Mi2-positive DM.

Figure 3. Correlation of the most differentially specifically overexpressed genes in patients with anti-Mi2-positive dermatomyositis and: titer of anti-Mi2 autoantibodies by ELISA (Mi2t), type 1 interferon-inducible genes (ISG15, MX1), type 2 interferon-inducible genes (GBP2, IFI30), T-cell markers (CD3E, CD4, CD8), macrophages (CD14, CD68), markers of muscle differentiation (NCAM1, MYOG, PAX7, MYH3, MYH8), and structural mature muscle proteins (ACTA1, MYH1, MYH2).
I. Pinal-Fernandez: None; J. Milisenda: None; K. Pak: None; S. Muñoz-Braceras: None; M. Casal-Dominguez: None; J. Torres-Ruiz: None; S. Dell´Orso: None; F. Naz: None; G. Gutierrez-Cruz: None; Y. Duque-Jaimez: None; A. Matas-Garcia: None; J. Padrosa: None; F. Garcia-Garcia: None; M. Guitart-Manpel: None; G. Garrabou: None; E. Trallero-Araguas: None; B. Wallit: None; J. Paik: None; J. Albayda: Amgen, 5, Janssen, 5; L. Christopher-Stine: None; T. Lloyd: None; J. Grau: None; A. Selva-O’Callaghan: None; A. Mammen: None.



