Abstract Session
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: PsA (1687–1692)
1692: Insights on the Use of JAK-inhibitors in Patients with Psoriatic Arthritis in an International Collaboration of Registers (the “JAK-pot” Study)
Monday, November 13, 2023
5:15 PM - 5:25 PM PT
Location: Ballroom 20A
Presenting Author(s)
Romain Aymon1, Denis Mongin2, Burkhard Leeb3, Monika Mustak-Blagusz4, Jakub Závada5, Karel Pavelka6, Dan Nordstrom7, Nina Trokovic8, Florenzo Iannone9, Catalin Codreanu10, Ziga Rotar11, Tore Kvien12, Sella Provan13, Manuel Enrique Pombo Suarez14, Fernando Alonso15, Fatos Onen16, Nevsun Inanc17, Louis Coupal18, Denis Choquette19, Galina Lukina20, Ori Elkayam21, Victoria Furer21, Ana Maria Rodrigues22, Delphine COURVOISIER23, Axel Finckh24, Michael Nissen2 and Kim Lauper25, 1Geneva University Hospitals, Collonges-sous-Salève, France, 2Geneva University Hospitals, Geneva, Switzerland, 3BioReg, Stockerau, Austria, 4BioReg, Vienna, Austria, 5Institute of Rheumatology, Prague, Czech Republic, 6Institut of Rheumatology and Department of Rheumatology, First Faculty of Medicine, Charles University, Praha, Czech Republic, 7Helsinki University Hospital, Helsinki, Finland, 8University of Helsinki, Helsinki, Finland, 9Rheumatology Unit, Department of Precision and Regenerative Medicine and Ionian Area, University of Bari "Aldo Moro", Bari, Italy, 10Center for Rheumatic Diseases, Bucharest, Romania, 11University Medical Centre Ljubljana, Ljubljana, Slovenia, 12Center for Treatment of Rheumatic and Musculoskeletal Diseases (REMEDY), Diakonhjemmet Hospital, Oslo, Norway, 13Diakonhjemmet Hospital, Oslo, Norway, 14Hospital Clínico Universitario, Santiago de Compostela, Spain, 15Spanish Society of Rheumatology, Madrid, Spain, 16Division of Rheumatology, Dokuz Eylul University School of Medicine, Izmir, Turkey, 17Division of Rheumatology, Medical School, Marmara University, Istanbul, Turkey, 18Institut de Rhumatologie de Montréal, Montreal, QC, Canada, 19Centre hospitalier de l'Université de Montréal (CHUM), Université de Montréal, Montréal, QC, Canada, 20Federal state budgetary scientific institution "V.A. Nasonova Research Institute of Rheumatology", Moscow, Russia, 21Tel Aviv Medical Center, Tel Aviv, Israel, 22Sociedade Portuguesa de Reumatologia; Nova Medical School; Hospital dos Lusíadas, Lisbon, Portugal, 23Division of Rheumatology, Geneva University Hospitals, Geneva, Switzerland, 24HUG, Geneva, Switzerland, 25Geneva University Hospitals, Genève, Switzerland
Background/Purpose: JAK-inhibitors (JAKi) are increasingly being prescribed to treat various inflammatory conditions, including psoriatic arthritis (PsA). While the understanding of JAKi efficacy and safety in rheumatoid arthritis is progressing, it remains less developed for PsA. The purpose of this study was to evaluate the profile of PsA patients to whom JAKi are prescribed, in a large multi-country real-world collaboration (JAK-pot).
Methods: Patients with a diagnosis of PsA, treated with either JAKi, TNF-inhibitors (TNFi) or bDMARDs with other modes of action (OMA) were included from 10 registers in which JAKi were prescribed for PsA. Treatment-courses were included only since JAKi became available in each country. We used standard descriptive statistics to evaluate patient-, disease-, and treatment characteristics across treatment groups, and across registers for JAKi only. We plotted crude retention rates for each treatment group.
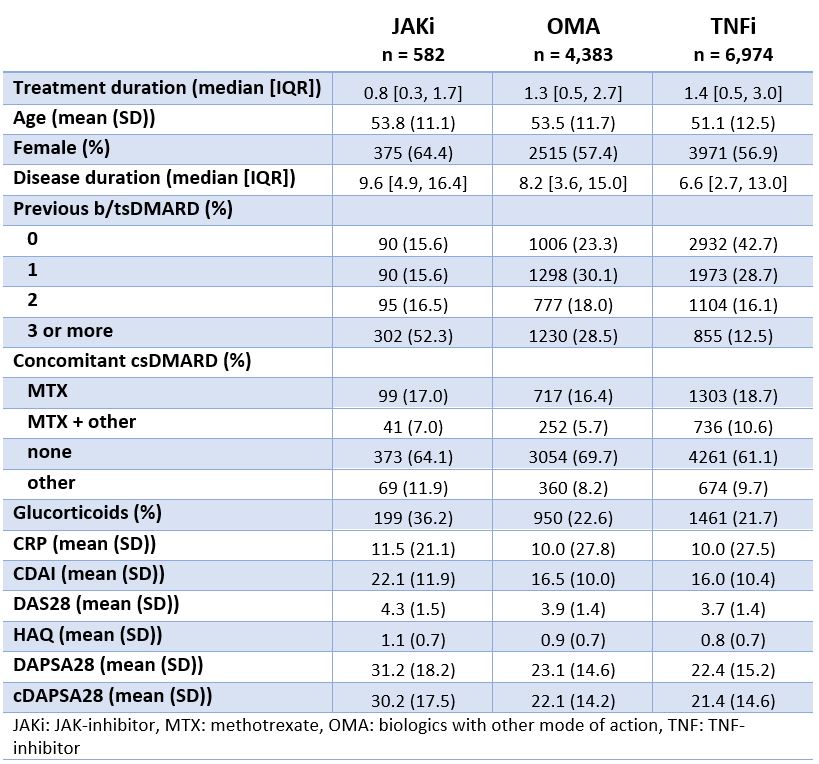
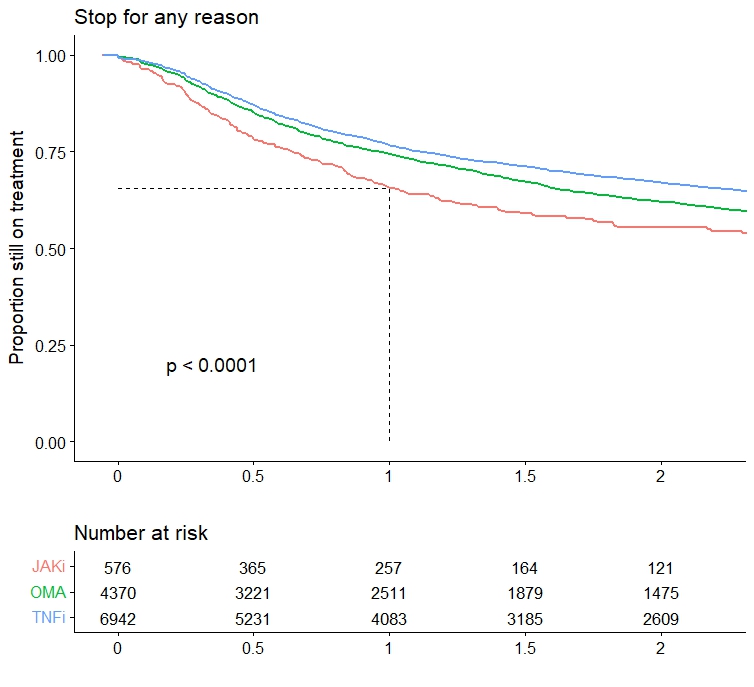
Results: Among the 11,939 treatment courses considered (Table 1), 582 were JAKi, mainly tofacitinib (67%) and upadacitinib (27%), and to a lesser extent baricitinib (6%). Patients initiating JAKi tended to have more difficult to treat disease, defined as longer disease duration ( > 9 years), older age, more prior bDMARD experience (52% with 3 or more previous bDMARDs), and more concomitant glucocorticoids. JAKi patient characteristics were consistent across countries (Table 2), despite varying use of specific JAKi agents, tofacitinib use ranging from 36 to 100% and upadacitinib from 0 to 64%. Crude drug retention at 1 year was 65% for JAKi was (Figure 1), which was significantly lower than for OMA (74%) and TNFi (77%).
Conclusion: Unadjusted drug retention rates for second line therapies in PsA patients suggest lower drug maintenance of JAKi compared to OMA and TNFi. However, it is likely that these results are largely driven by the severity of the disease of patients on JAKi compared to patients on other treatments. Adjusted analyses are needed when evaluating the real-life effectiveness and safety of JAKi for PsA patients.
.jpg)
R. Aymon: None; D. Mongin: None; B. Leeb: AbbVie/Abbott, 2, Biogen, 2, 6, Celgene, 2, 6, Eli Lilly, 2, 6, Pfizer, 2, 6; M. Mustak-Blagusz: None; J. Závada: AbbVie/Abbott, 2, 6, Egis, 2, 6, Eli Lilly, 2, 6, Novartis, 2, 6, Sandoz, 2, 6, UCB, 2, 6; K. Pavelka: Abbvie, 2, 6, Amgen, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Egis, 2, 6, MSD, 2, 6, Pfizer, 2, 6, Roche, 2, 6, UCB, 2, 6; D. Nordstrom: AbbVie/Abbott, 2, BMS, 2, Lilly, 2, MSD, 2, Novartis, 2, Pfizer, 2, UCB, 2; N. Trokovic: None; F. Iannone: Abbvie, 2, 5, BMS, 2, 5, Janssen, 2, 5, Lilly, 2, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Roche, 2, 5, UCB, 2, 5; C. Codreanu: AbbVie/Abbott, 2, 6, Amgen, 1, 6, Boehringer-Ingelheim, 1, 6, Eli Lilly, 1, 6, Novartis, 1, 6, Pfizer, 1, 6; Z. Rotar: None; T. Kvien: AbbVie/Abbott, 1, 2, 6, Bristol-Myers Squibb(BMS), 5, Galapagos, 2, 5, Gilead, 2, grunenthal, 6, Janssen, 2, 6, Novartis, 5, Pfizer, 2, 5, sandoz, 2, 6, UCB, 2, 5, 6; S. Provan: None; M. Pombo Suarez: Janssen, 6, MSD Spain, 6; F. Alonso: None; F. Onen: AbbVie/Abbott, 6, Amgen, 6, Novartis, 6, Pfizer, 6, UCB, 6; N. Inanc: AbbVie/Abbott, 2, 6, Abdi Ibrahim, 2, 6, Amgen, 2, 6, Boehringer-Ingelheim, 2, 6, Eli Lilly, 2, 6, Merck/MSD, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 2, 6; L. Coupal: None; D. Choquette: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Eli Lilly, 2, 5, 6, Fresenius-Kabi, 2, 5, 6, JAMP pharma, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sandoz, 2, 5, 6, Tevapharm, 2, 5, 6; G. Lukina: None; O. Elkayam: None; V. Furer: None; A. Rodrigues: AbbVie/Abbott, 5, Amgen, 5, 6, Novartis, 5, Pfizer, 5; D. COURVOISIER: None; A. Finckh: None; M. Nissen: AbbVie/Abbott, 2, Eli Lilly, 2, 12, Involved in Clinical Trial, Janssen, 2, Novartis, 6, 12, research funding paid to institution, Pfizer, 6, UCB, 2, 12, funding support to attend EULAR 2023, paid to institution; K. Lauper: Eli Lilly, 5, Pfizer, 2.
Background/Purpose: JAK-inhibitors (JAKi) are increasingly being prescribed to treat various inflammatory conditions, including psoriatic arthritis (PsA). While the understanding of JAKi efficacy and safety in rheumatoid arthritis is progressing, it remains less developed for PsA. The purpose of this study was to evaluate the profile of PsA patients to whom JAKi are prescribed, in a large multi-country real-world collaboration (JAK-pot).
Methods: Patients with a diagnosis of PsA, treated with either JAKi, TNF-inhibitors (TNFi) or bDMARDs with other modes of action (OMA) were included from 10 registers in which JAKi were prescribed for PsA. Treatment-courses were included only since JAKi became available in each country. We used standard descriptive statistics to evaluate patient-, disease-, and treatment characteristics across treatment groups, and across registers for JAKi only. We plotted crude retention rates for each treatment group.
Results: Among the 11,939 treatment courses considered (Table 1), 582 were JAKi, mainly tofacitinib (67%) and upadacitinib (27%), and to a lesser extent baricitinib (6%). Patients initiating JAKi tended to have more difficult to treat disease, defined as longer disease duration ( > 9 years), older age, more prior bDMARD experience (52% with 3 or more previous bDMARDs), and more concomitant glucocorticoids. JAKi patient characteristics were consistent across countries (Table 2), despite varying use of specific JAKi agents, tofacitinib use ranging from 36 to 100% and upadacitinib from 0 to 64%. Crude drug retention at 1 year was 65% for JAKi was (Figure 1), which was significantly lower than for OMA (74%) and TNFi (77%).
Conclusion: Unadjusted drug retention rates for second line therapies in PsA patients suggest lower drug maintenance of JAKi compared to OMA and TNFi. However, it is likely that these results are largely driven by the severity of the disease of patients on JAKi compared to patients on other treatments. Adjusted analyses are needed when evaluating the real-life effectiveness and safety of JAKi for PsA patients.

Table1: Baseline characteristics across treatment groups
.jpg)
Table 2: Baseline characteristics for JAK inhibitors across countries

Figure 1: Crude drug retention rates
R. Aymon: None; D. Mongin: None; B. Leeb: AbbVie/Abbott, 2, Biogen, 2, 6, Celgene, 2, 6, Eli Lilly, 2, 6, Pfizer, 2, 6; M. Mustak-Blagusz: None; J. Závada: AbbVie/Abbott, 2, 6, Egis, 2, 6, Eli Lilly, 2, 6, Novartis, 2, 6, Sandoz, 2, 6, UCB, 2, 6; K. Pavelka: Abbvie, 2, 6, Amgen, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Egis, 2, 6, MSD, 2, 6, Pfizer, 2, 6, Roche, 2, 6, UCB, 2, 6; D. Nordstrom: AbbVie/Abbott, 2, BMS, 2, Lilly, 2, MSD, 2, Novartis, 2, Pfizer, 2, UCB, 2; N. Trokovic: None; F. Iannone: Abbvie, 2, 5, BMS, 2, 5, Janssen, 2, 5, Lilly, 2, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Roche, 2, 5, UCB, 2, 5; C. Codreanu: AbbVie/Abbott, 2, 6, Amgen, 1, 6, Boehringer-Ingelheim, 1, 6, Eli Lilly, 1, 6, Novartis, 1, 6, Pfizer, 1, 6; Z. Rotar: None; T. Kvien: AbbVie/Abbott, 1, 2, 6, Bristol-Myers Squibb(BMS), 5, Galapagos, 2, 5, Gilead, 2, grunenthal, 6, Janssen, 2, 6, Novartis, 5, Pfizer, 2, 5, sandoz, 2, 6, UCB, 2, 5, 6; S. Provan: None; M. Pombo Suarez: Janssen, 6, MSD Spain, 6; F. Alonso: None; F. Onen: AbbVie/Abbott, 6, Amgen, 6, Novartis, 6, Pfizer, 6, UCB, 6; N. Inanc: AbbVie/Abbott, 2, 6, Abdi Ibrahim, 2, 6, Amgen, 2, 6, Boehringer-Ingelheim, 2, 6, Eli Lilly, 2, 6, Merck/MSD, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 2, 6; L. Coupal: None; D. Choquette: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Eli Lilly, 2, 5, 6, Fresenius-Kabi, 2, 5, 6, JAMP pharma, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sandoz, 2, 5, 6, Tevapharm, 2, 5, 6; G. Lukina: None; O. Elkayam: None; V. Furer: None; A. Rodrigues: AbbVie/Abbott, 5, Amgen, 5, 6, Novartis, 5, Pfizer, 5; D. COURVOISIER: None; A. Finckh: None; M. Nissen: AbbVie/Abbott, 2, Eli Lilly, 2, 12, Involved in Clinical Trial, Janssen, 2, Novartis, 6, 12, research funding paid to institution, Pfizer, 6, UCB, 2, 12, funding support to attend EULAR 2023, paid to institution; K. Lauper: Eli Lilly, 5, Pfizer, 2.




