Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes II: Omics (1693–1698)
1698: High-throughput Proteomics Identities a Spectrum of Novel Serum Biomarkers of Lupus Nephritis
Monday, November 13, 2023
5:15 PM - 5:25 PM PT
Location: Room 6F/6C

Huihua Ding, MD
Shanghai Jiao Tong University School of Medicine affiliated Renji Hospital
Shanghai, Shanghai, ChinaDisclosure information not submitted.
Presenting Author(s)
Huihua Ding, Yiwei Shen, Min Dai and Nan Shen, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background/Purpose: Navigating the molecular complexity of lupus nephritis (LN), a heterogeneous autoimmune disorder, poses challenges to biomarker discovery. This study addresses these challenges through an integrative approach, combining high-throughput proteomics and curated clinical samples from diverse LN states and subtypes. Our goal is to identify novel biomarkers, enhancing our understanding of LN and informing personalized treatments.
Methods: Proximity extension immunoassay (PEA, Olink) was used to evaluate the serum levels of inflammation related proteins using Explore 384-plex panel in the cohort of 80 SLE patients, including 64 active LN patients with concurrent renal biopsies and 16 active SLE patients without renal involvement at the baseline, as well as 8 age-and sex- matched healthy controls (HCs) (Table 1). Ontological and interrelationship analyses between proteins were performed by GEO, KEGG and String analyses, to interpret the high-throughput proteomic data.
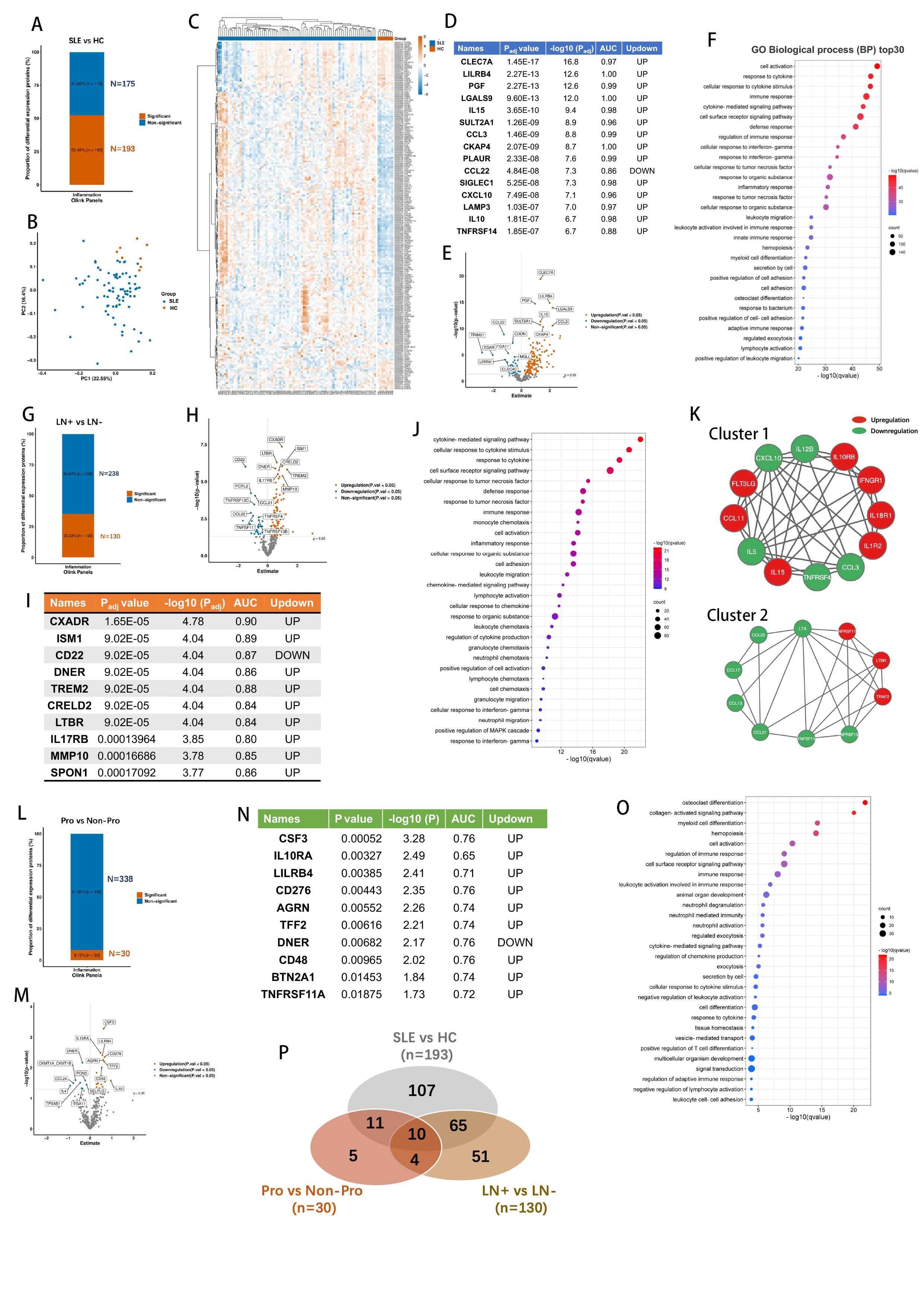
Results: Among the 368 molecules analyzed, 193 differentially expressed serum protein signatures were identified by the comparative analysis between SLE patients and HCs including 144 increased and 49 decreased molecules in SLE patients compared to HCs (Figure 1A-E). The Gene Ontology (GO) analysis was performed to enrich the primary biological processes (Figure 1F). Further comparative analysis was performed in the 80 SLE patients to identify novel biomarkers for kidney damage. There were 130 differentially expressed proteins between LN patients and non-LN SLE patients (Figure 1G-I). The top biological processes were enriched in cytokine-mediated signaling pathway, cellular response to cytokine stimulus, response to cytokine and cell surface receptor signaling pathway (Figure 1J). MCODE module screening using String software identified two clusters among the differential expressed proteins (Figure 1K). Importantly, for the 64 biopsy-proven LN patients, 30 circulating proteins were found significantly altered between proliferative LN (III±V and IV±V) and membranous LN (V) patients (Figure 1L-N). The GO analysis demonstrated osteoclast differentiation, collagen-activated signaling pathway, myeloid cell differentiation and hemopoiesis were enriched in the differential proteins (Figure 1O). Venn diagram was performed to illustrate the distribution of total differential expressed proteins of the three comparasion sets (Figure 1P).
Conclusion: The combination of high-throughput proteomics and well-structured clinical samples provides the foundation to dissect the molecular complexity of LN, potentially leading to the discovery and validation of effective biomarkers for this challenging disease.
H. Ding: None; Y. Shen: None; M. Dai: None; N. Shen: None.
Background/Purpose: Navigating the molecular complexity of lupus nephritis (LN), a heterogeneous autoimmune disorder, poses challenges to biomarker discovery. This study addresses these challenges through an integrative approach, combining high-throughput proteomics and curated clinical samples from diverse LN states and subtypes. Our goal is to identify novel biomarkers, enhancing our understanding of LN and informing personalized treatments.
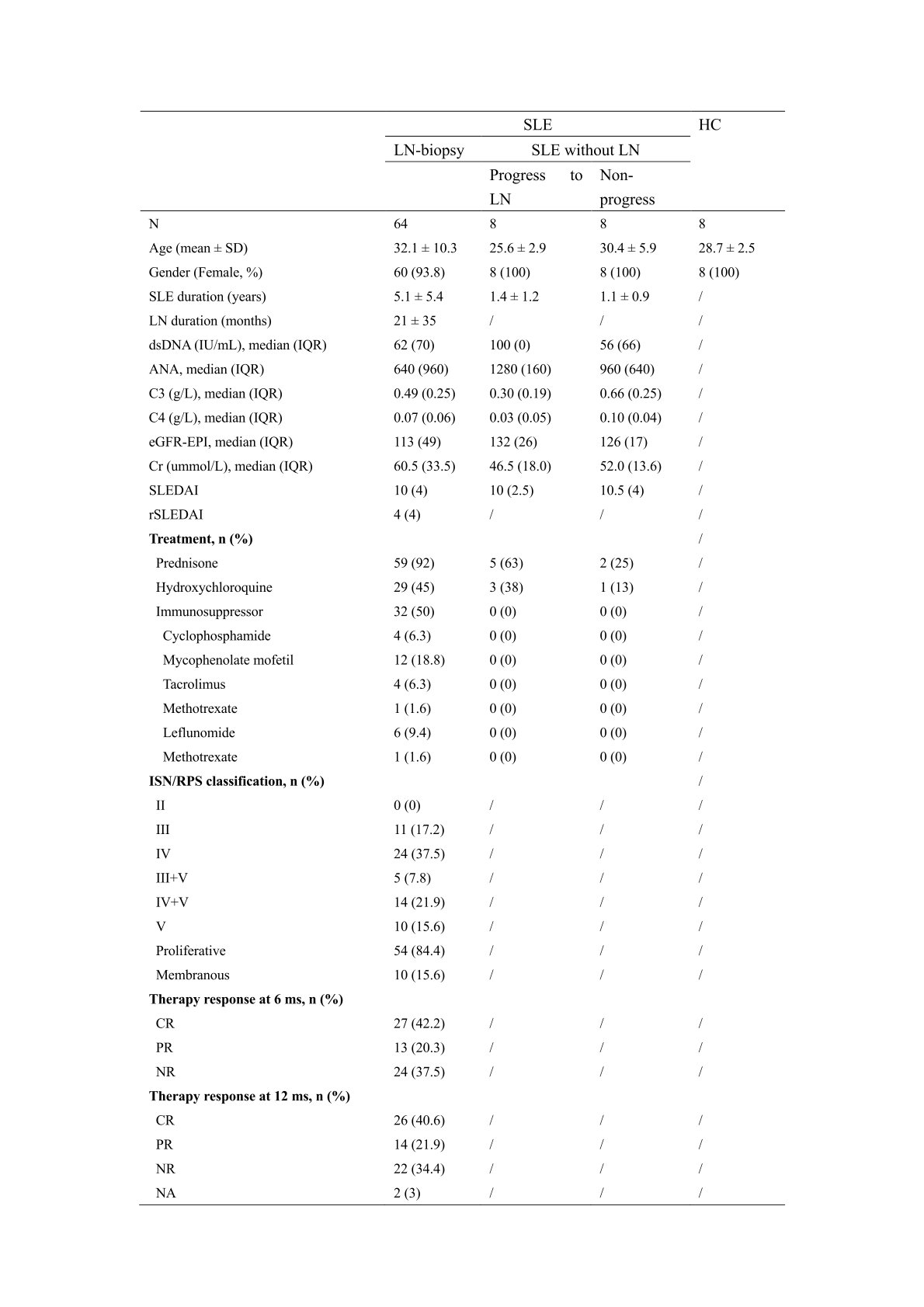
Methods: Proximity extension immunoassay (PEA, Olink) was used to evaluate the serum levels of inflammation related proteins using Explore 384-plex panel in the cohort of 80 SLE patients, including 64 active LN patients with concurrent renal biopsies and 16 active SLE patients without renal involvement at the baseline, as well as 8 age-and sex- matched healthy controls (HCs) (Table 1). Ontological and interrelationship analyses between proteins were performed by GEO, KEGG and String analyses, to interpret the high-throughput proteomic data.
Results: Among the 368 molecules analyzed, 193 differentially expressed serum protein signatures were identified by the comparative analysis between SLE patients and HCs including 144 increased and 49 decreased molecules in SLE patients compared to HCs (Figure 1A-E). The Gene Ontology (GO) analysis was performed to enrich the primary biological processes (Figure 1F). Further comparative analysis was performed in the 80 SLE patients to identify novel biomarkers for kidney damage. There were 130 differentially expressed proteins between LN patients and non-LN SLE patients (Figure 1G-I). The top biological processes were enriched in cytokine-mediated signaling pathway, cellular response to cytokine stimulus, response to cytokine and cell surface receptor signaling pathway (Figure 1J). MCODE module screening using String software identified two clusters among the differential expressed proteins (Figure 1K). Importantly, for the 64 biopsy-proven LN patients, 30 circulating proteins were found significantly altered between proliferative LN (III±V and IV±V) and membranous LN (V) patients (Figure 1L-N). The GO analysis demonstrated osteoclast differentiation, collagen-activated signaling pathway, myeloid cell differentiation and hemopoiesis were enriched in the differential proteins (Figure 1O). Venn diagram was performed to illustrate the distribution of total differential expressed proteins of the three comparasion sets (Figure 1P).
Conclusion: The combination of high-throughput proteomics and well-structured clinical samples provides the foundation to dissect the molecular complexity of LN, potentially leading to the discovery and validation of effective biomarkers for this challenging disease.

Table 1. Demographic and clinical characteristics of study subjects (n=88).

Figure 1: (A-F) Comparison of protein expression between SLE patients and healthy controls (HC). (A) There were 193 differentially expressed proteins including 144 increased and 49 decreased molecules in SLE compared to HCs; (B) PCA analysis revealed the distinctive distribution for the two groups; (C) Heat map of protein expression profiles in SLE and HCs; (D-E) The top 15 differentially expressed proteins were listed and plotted; (F) The Gene Ontology (GO) analysis was performed to identify the top biological processes for the SLE vs HC set. (G-K) Comparison of protein expression between LN patients and non-LN SLE patients; (G) There were 130 differentially expressed proteins in LN patients compared to non-LN patients. (H-I) The top 10 differentially expressed proteins were listed and plotted. (J) The Gene Ontology (GO) analysis was performed to identify the top biological processes for the LN vs Non-LN set. (K) The MCODE module screening were performed identifying two clusters using the differentially expressed proteins in the String software. (L-O) Comparison of protein expression between proliferative LN (III±V and IV±V) and membranous (V) LN patients. (L-M) There were 30 differentially expressed proteins in the proliferative LN compared to membranous LN groups; (N) The top 10 differentially expressed proteins were listed; (O) The Gene Ontology (GO) analysis was performed to identify the top biological processes for the proliferative vs membranous LN set. (P) Venn plot illustrated the distribution of the total differentially expressed proteins in three comparison sets.
H. Ding: None; Y. Shen: None; M. Dai: None; N. Shen: None.



