Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes II: Omics (1693–1698)
1697: Molecular Characterisation of Remission and Lupus Low Disease Activity State (LLDAS) by Whole-Blood Transcriptome-Based Pathways in a Pan-European Systemic Lupus Erythematosus Cohort
Monday, November 13, 2023
5:00 PM - 5:10 PM PT
Location: Room 6F/6C

Ioannis Parodis, MD, PhD
Karolinska Institutet
Stockholm, SwedenDisclosure information not submitted.
Presenting Author(s)
Ioannis Parodis1, Julius Lindblom1, Guillermo Barturen2, Rafaela Ortega Castro3, Ricard Cervera4, Jacques-Olivier Pers5, Fernanda Genre Romero6, Falk Hiepe7, Maria Gerosa8, Laszlo Kovacs9, Ellen De Langhe10, Silvia Piantoni11, Georg Stummvoll12, Carlos Vasconcelos13, Barbara Vigone14, Torsten Witte15, Marta Alarcon-Riquelme16 and Lorenzo Beretta17, 1Karolinska Institutet, Stockholm, Sweden, 2Center for Genomics and Oncological Research (GENYO), Andalusia, Spain, 3Hospital Reina Sofía, Cordoba, Spain, 4Hospital Clínic de Barcelona, Barcelona, Spain, 5Centre Hospitalier Universitaire de Brest, Hospital de la Cavale Blanche, Brest, France, 6IDIVAL, Santander, Spain, 7Charité Universitatsmedizine - Berlin, Berlin, Germany, 8University of Milan, Milano, Italy, 9University of Szeged, Szeged, Hungary, 10Katholieke Universiteit Leuven and Universitair Ziekenhuis Leuven, Leuven, Belgium, 11ASST Spedali Civili and University of Brescia, Brescia, Italy, 12Medical University Vienna, Baden, Austria, 13Centro Hospitalar do Porto, Porto, Portugal, 14Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano, Italy, 15Dept of Immunology and Rheumatology, Hannover, Germany, 16Center for Genomics and Oncological Research (GENYO), Granada, Spain, 17Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico di MIlano, Milan, Italy
Background/Purpose: Treating to remission or lupus low disease activity state (LLDAS) are conceptual frameworks for the management of SLE, but the biological milieus underlying these states have yet to be explored. We aimed at determining differentially expressed pathways (DEPs) between remission and non-remission state as well as between LLDAS and non-LLDAS.
Methods: Patients with SLE from the PRECISESADS project (NTC02890121) were stratified into patients fulfilling and not fulfilling the criteria of (i) DORIS remission, (ii) LLDAS, and (iii) LLDAS after exclusion of remission attainers. None of the patients had been treated with cyclophosphamide within 6 months or depletive therapies within 12 months from baseline.
Results: We had available data from 321 patients; 17.4% were in DORIS remission and 40.8% were in LLDAS; 28.3% of non-remission patients were in LLDAS. A total of 1465 unique Reactome pathways were selected for analysis. Overall, 288 pathways differed significantly between DORIS remitters and non-remitters with an FDR-corrected p (q)< 0.05 and a robust effect size (dr)≥0.36; of those, 97 were downregulated and 191 upregulated in DORIS remitters. Clustering of significant pathways yielded 3 distinct groups of patients. Accordingly, 604 pathways differed significantly (q< 0.05 and dr≥0.36) in LLDAS vs. non-LLDAS patients; 226 pathways were downregulated and 378 upregulated in patients in LLDAS. After clustering, 3 distinct groups could be identified (separation of classes among clusters, χ2=25.3; p< 0.001). In both cases, the 3 clusters were characterized by differential serological, musculoskeletal and renal activity, as well as use of immunosuppressants. Analysis of adjacent levels of disease activity using forward difference coding in linear regression models showed no DEPs between patients in DORIS remission compared with patients in LLDAS after suppression of the remitting patients. By contrast, 662 DEPs were documented between patients in LLDAS after suppression of the remitting patients and non-LLDAS patients.
Conclusion: We demonstrated for the first time molecular signaling pathways distinguishing remission/LLDAS from active SLE. Remission/LLDAS was associated with reversal of biological processes related to SLE pathogenesis, and processes linked to specific clinical manifestations. While DEP clustering by DORIS remission better grouped patients than clustering by LLDAS, substantiating the conceptual testimonial of remission being the ultimate treatment goal in SLE, the lack of substantial pathway differentiation between the two states justifies LLDAS as an acceptable goal from a biological perspective when remission is not achievable. The study revealed potentiality of existing drugs that could be repurposed to treat SLE and important pathways underlying active SLE whose modulation could aid attainment of remission. Among those, TLR cascades, BTK activity, the CTLA-4-related inhibitory signaling, and the NLRP3 inflammasome pathway were of particular interest.
I. Parodis: Amgen, 5, 6, AstraZeneca, 5, 6, Aurinia Pharmaceuticals, 5, 6, Bristol-Myers Squibb(BMS), 5, 6, Elli Lilly and Company, 5, 6, F. Hoffmann-La Roche AG, 5, 6, Gilead Sciences, 5, 6, GSK, 5, 6, Janssen Pharmaceuticals, 5, 6, Novartis, 5, 6, Otsuka Pharmaceutical, 5, 6; J. Lindblom: None; G. Barturen: None; R. Ortega Castro: None; R. Cervera: None; J. Pers: None; F. Genre Romero: None; F. Hiepe: None; M. Gerosa: None; L. Kovacs: None; E. De Langhe: None; S. Piantoni: None; G. Stummvoll: None; C. Vasconcelos: None; B. Vigone: None; T. Witte: None; M. Alarcon-Riquelme: None; L. Beretta: None.
Background/Purpose: Treating to remission or lupus low disease activity state (LLDAS) are conceptual frameworks for the management of SLE, but the biological milieus underlying these states have yet to be explored. We aimed at determining differentially expressed pathways (DEPs) between remission and non-remission state as well as between LLDAS and non-LLDAS.
Methods: Patients with SLE from the PRECISESADS project (NTC02890121) were stratified into patients fulfilling and not fulfilling the criteria of (i) DORIS remission, (ii) LLDAS, and (iii) LLDAS after exclusion of remission attainers. None of the patients had been treated with cyclophosphamide within 6 months or depletive therapies within 12 months from baseline.
Results: We had available data from 321 patients; 17.4% were in DORIS remission and 40.8% were in LLDAS; 28.3% of non-remission patients were in LLDAS. A total of 1465 unique Reactome pathways were selected for analysis. Overall, 288 pathways differed significantly between DORIS remitters and non-remitters with an FDR-corrected p (q)< 0.05 and a robust effect size (dr)≥0.36; of those, 97 were downregulated and 191 upregulated in DORIS remitters. Clustering of significant pathways yielded 3 distinct groups of patients. Accordingly, 604 pathways differed significantly (q< 0.05 and dr≥0.36) in LLDAS vs. non-LLDAS patients; 226 pathways were downregulated and 378 upregulated in patients in LLDAS. After clustering, 3 distinct groups could be identified (separation of classes among clusters, χ2=25.3; p< 0.001). In both cases, the 3 clusters were characterized by differential serological, musculoskeletal and renal activity, as well as use of immunosuppressants. Analysis of adjacent levels of disease activity using forward difference coding in linear regression models showed no DEPs between patients in DORIS remission compared with patients in LLDAS after suppression of the remitting patients. By contrast, 662 DEPs were documented between patients in LLDAS after suppression of the remitting patients and non-LLDAS patients.
Conclusion: We demonstrated for the first time molecular signaling pathways distinguishing remission/LLDAS from active SLE. Remission/LLDAS was associated with reversal of biological processes related to SLE pathogenesis, and processes linked to specific clinical manifestations. While DEP clustering by DORIS remission better grouped patients than clustering by LLDAS, substantiating the conceptual testimonial of remission being the ultimate treatment goal in SLE, the lack of substantial pathway differentiation between the two states justifies LLDAS as an acceptable goal from a biological perspective when remission is not achievable. The study revealed potentiality of existing drugs that could be repurposed to treat SLE and important pathways underlying active SLE whose modulation could aid attainment of remission. Among those, TLR cascades, BTK activity, the CTLA-4-related inhibitory signaling, and the NLRP3 inflammasome pathway were of particular interest.

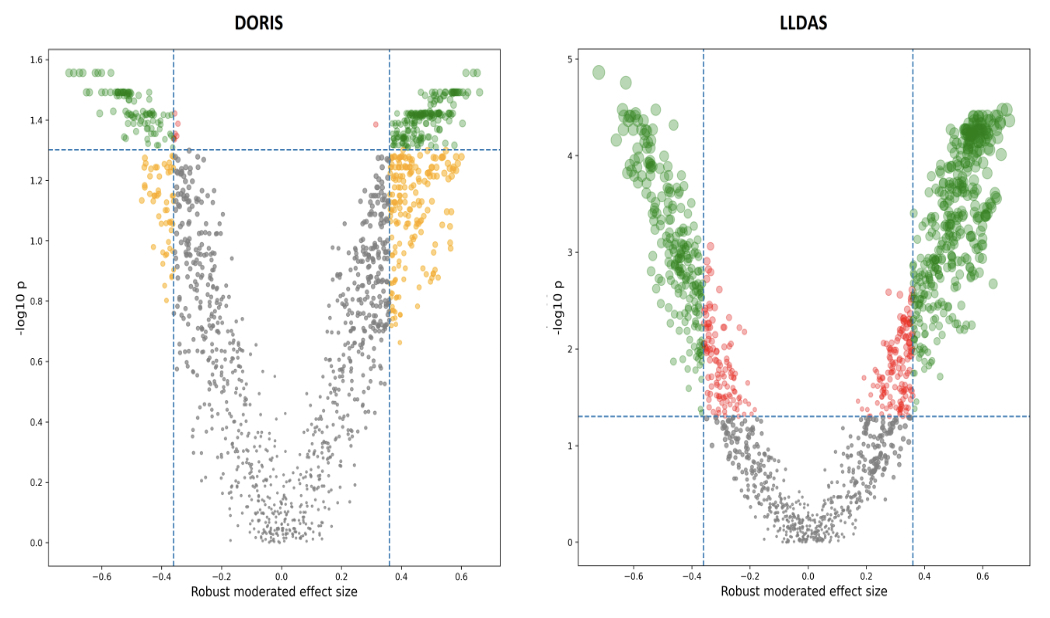
Figure 1. Volcano plot in DORIS and LLDAS subjects. Volcano plot of Reactome pathways in DORIS (left panel) or LLDAS patients (right panel). The horizontal dashed line indicates the log-transformed false-discovery rate probability threshold (q=0.05) for the moderated t-test statistic; the vertical dashed lines indicate the moderated robust effect size threshold (|dr|=0.36). Significant pathways for both conditions are highlighted in green. DORIS, definition of remission in systemic lupus erythematosus; LLDAS, lupus low disease activity state.

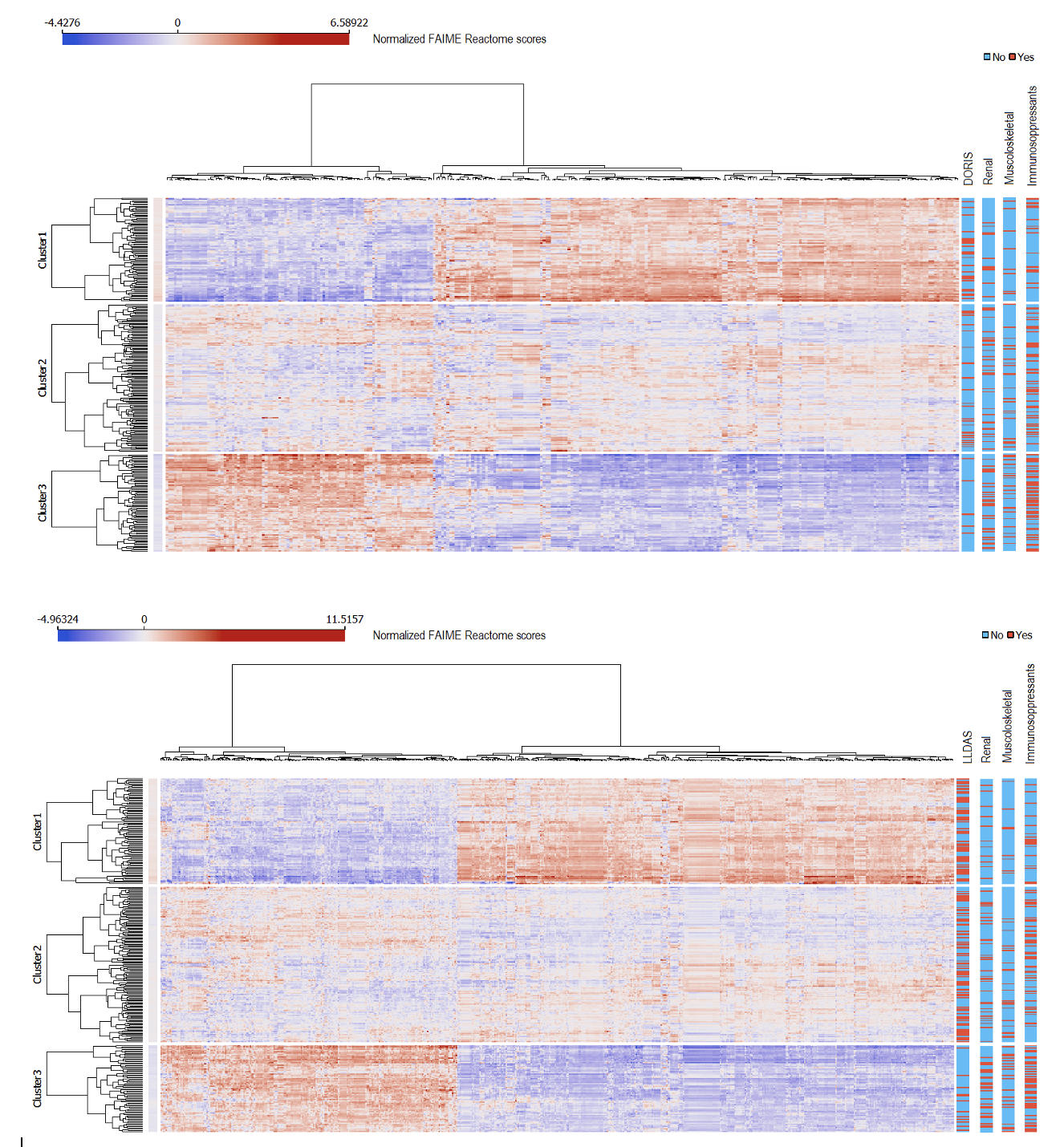
Figure 2. Clusters of Reactome individualized pathways. Individualized Reactome pathways after clustering of selected features associated with DORIS remission (top panel) or LLDAS (bottom panel). The bars to the right illustrate the distribution of relevant clinical features across clusters. DORIS, definitions of remission in systemic lupus erythematosus; LLDAS, lupus low disease activity state. Cluster1, DORIS/LLDAS cluster; Cluster2, mixed cluster; Cluster3, non-remission/non-LLDAS cluster.

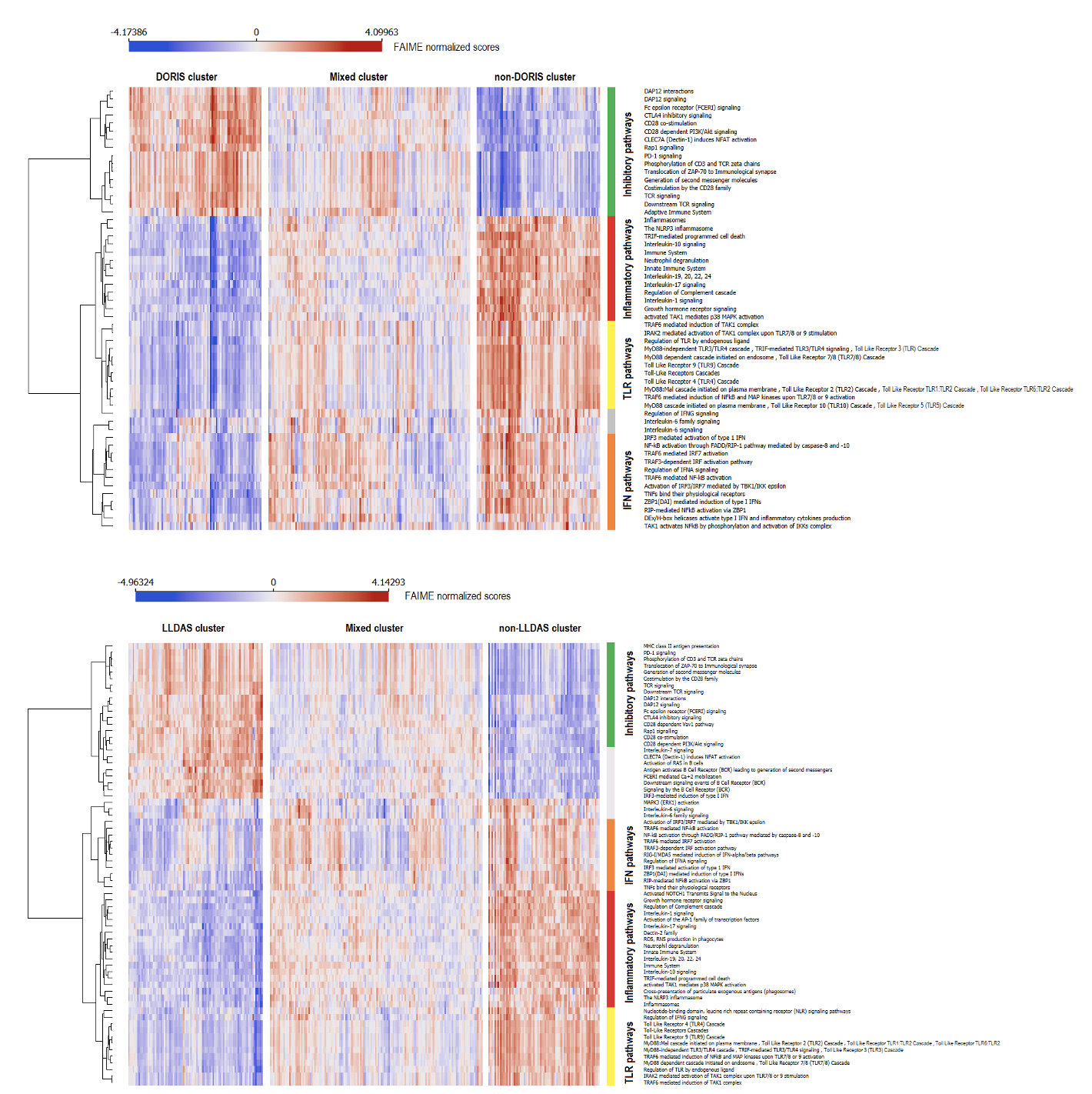
Figure 3. Immune system Rectome pathways according to biological clusters and main function. Distribution of individualized immune system Rectome pathways in DORIS (top panel) and LLDAS (bottom panel). The colored bars represent manual annotation according to the main functions of each pathway cluster, with green denoting pathways with inhibitory function on the immune system, red denoting inflammasome/inflammatory pathways enriched in cytokines, yellow denoting toll-like receptor (TLR) and related functions, orange denoting interferon (IFN) pathways, and grey denoting mixed, interleukin (IL)-6, B cells, or other pathways. DORIS, definitions of remission in systemic lupus erythematosus; LLDAS, lupus low disease activity state.
I. Parodis: Amgen, 5, 6, AstraZeneca, 5, 6, Aurinia Pharmaceuticals, 5, 6, Bristol-Myers Squibb(BMS), 5, 6, Elli Lilly and Company, 5, 6, F. Hoffmann-La Roche AG, 5, 6, Gilead Sciences, 5, 6, GSK, 5, 6, Janssen Pharmaceuticals, 5, 6, Novartis, 5, 6, Otsuka Pharmaceutical, 5, 6; J. Lindblom: None; G. Barturen: None; R. Ortega Castro: None; R. Cervera: None; J. Pers: None; F. Genre Romero: None; F. Hiepe: None; M. Gerosa: None; L. Kovacs: None; E. De Langhe: None; S. Piantoni: None; G. Stummvoll: None; C. Vasconcelos: None; B. Vigone: None; T. Witte: None; M. Alarcon-Riquelme: None; L. Beretta: None.



