Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes II: Omics (1693–1698)
1693: Blood Immunophenotyping Distinguishes Three Subgroups of Lupus Nephritis Patients with Distinct Kidney Infiltrates and Interferon Signatures
Monday, November 13, 2023
4:00 PM - 4:10 PM PT
Location: Room 6F/6C
- AH
Alice Horisberger, MD
Brigham and Woman's Hospital, Harvard medical school
Boston, MA, United StatesDisclosure information not submitted.
Presenting Author(s)
Alice Horisberger1, Alec Griffith1, Arnon Arazi2, Joshua Keegan1, Kaitlyn Howard1, Takanori Sasaki1, Tusharkanti Ghosh3, Andrea Fava4, Jun Inamo5, John Pulford1, Ekaterina Murzin1, Brandon Hancock1, Katie Preisinger6, Maria Gutierrez-Arcelus7, Thomas Eisenhaure8, Joel Guthridge9, Paul Hoover10, Maria Dall'Era11, David Wofsy11, Diane L. Kamen12, Kenneth Kalunian13, Richard Furie14, H Michael Belmont15, Peter Izmirly6, Robert Clancy16, David Hildeman17, steve Woodle17, William Apruzzese18, Maureen McMahon19, Jennifer Grossman20, Jennifer Barnas21, Fernanda Payan-Schober22, Mariko Ishimori23, Accelerating Medicines Partnership Program RA SLE Network23, Matthias Kretzler24, Celine Berthier24, Jeff Hodgin24, Dawit Demeke24, Chaim Putterman25, Nir Hacohen8, Michael Brenner1, Jennifer Anolik21, Anne Davidson26, Judith James9, Soumya Raychaudhuri10, Michelle Petri27, Jill Buyon16, Betty Diamond26, The Accelerating Medicines Partnership In RA/SLE28, Fan Zhang29, James Lederer1 and Deepak Rao10, 1Brigham and Women's Hospital and Harvard Medical School, Boston, MA, 2Broad Institute of MIT and Harvard, Melrose, MA, 3School of Public Health, University of Colorado, Anschutz Medical Campus, Aurora, CO, 4Johns Hopkins University, Baltimore, MD, 5University of Colorado School of Medicine, Aurora, CO, 6New York University School of Medicine, New York, NY, 7Boston Children's Hospital, Boston, MA, 8Broad Institute of MIT and Harvard, Cambridge, MA, 9Oklahoma Medical Research Foundation, Oklahoma City, OK, 10Brigham and Women's Hospital, Boston, MA, 11University of California San Francisco, San Francisco, CA, 12Medical University of South Carolina, Charleston, SC, 13University of California San Diego, La Jolla, CA, 14Northwell Health, Manhasset, NY, 15NYU School of Medicine, New York, NY, 16NYU Grossman School of Medicine, New York, NY, 17University of Cincinnati College of Medicine, Cincinnati, OH, 18Accelerating Medicines Partnership® Program: Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP® RA/SLE) Network, Boston, MA, 19UCLA David Geffen School of Medicine, Los Angeles, CA, 20University of California Los Angeles, Los Angeles, CA, 21University of Rochester Medical Center, Rochester, NY, 22Texas Tech University Health Sciences Center, El Paso, TX, 23Cedars-Sinai Medical Center, Los Angeles, CA, 24University of Michigan, Ann Arbor, MI, 25Albert Einstein College of Medicine, Bronx, NY, 26Feinstein Institutes for Medical Research, Manhasset, NY, 27Department of Medicine, Division of Rheumatology, Johns Hopkins University School of Medicine, Timonium, MD, 28multisites, multisites, 29University of Colorado, Aurora, CO
Background/Purpose: Patients with lupus nephritis (LN) have variable responses to standard-of-care therapy, and a third of patients with class III, IV, or V show a progressive decline in kidney function. Identifying distinct inflammatory processes associated with LN using non-invasive tools may improve treatment targeting. Here, we aimed to identify subgroups of LN patients that differ in systemic immune activity and to evaluate their relationship to kidney pathology.
Methods: Mass cytometry using four 48-marker panels was applied to characterize peripheral blood mononuclear cells from 140 patients with active, biopsy-proven proliferative (class III or IV +/- V, n=98) or membranous (class V, n=42) nephritis and 40 healthy controls in the Accelerated Medicine Partnership RA/SLE Network Phase II study. K-means clustering was used to stratify patients based on the proportions of 55 immune cell subsets defined using B cell-, T cell-, myeloid cell-, and NK cell-focused panels.
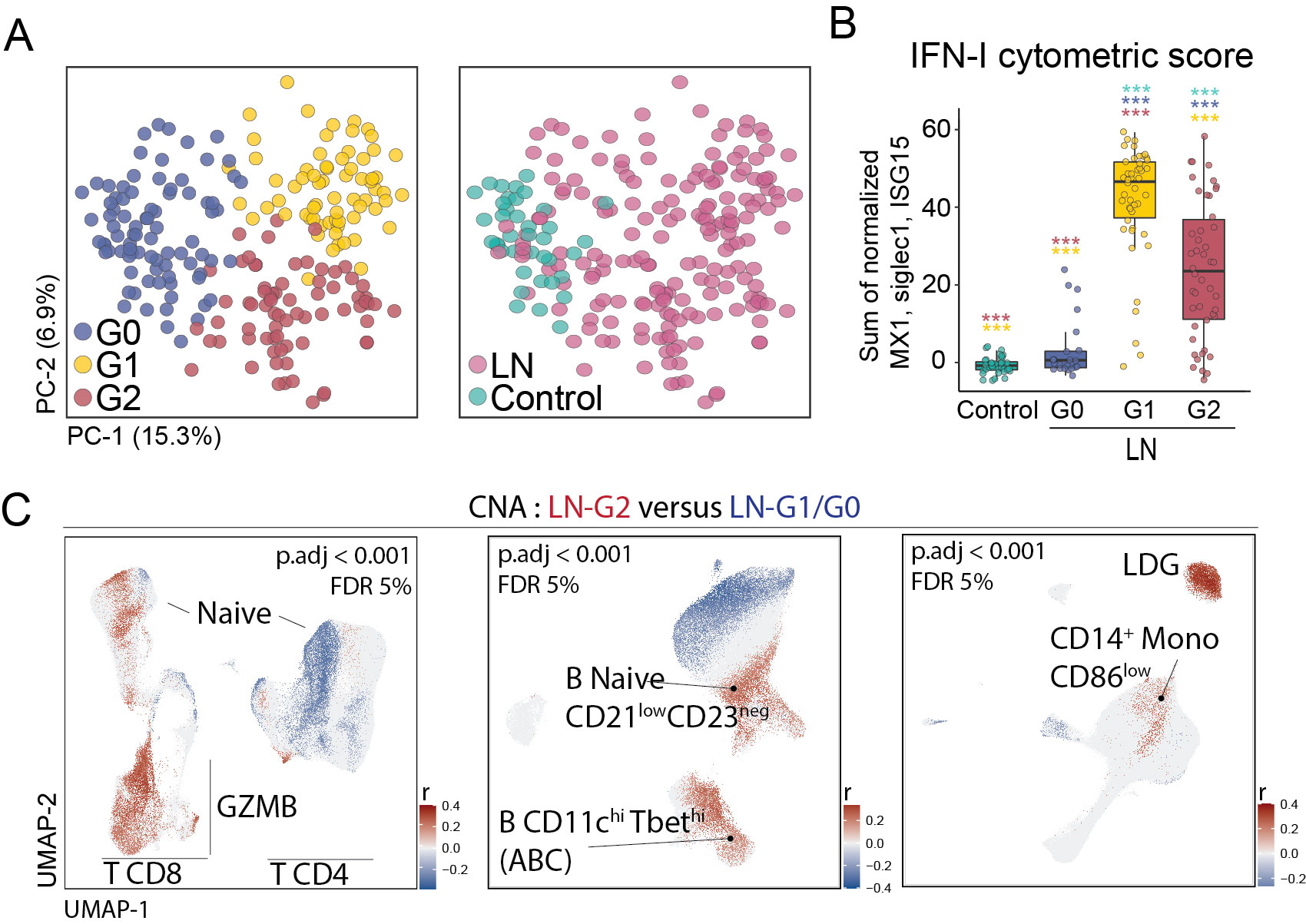
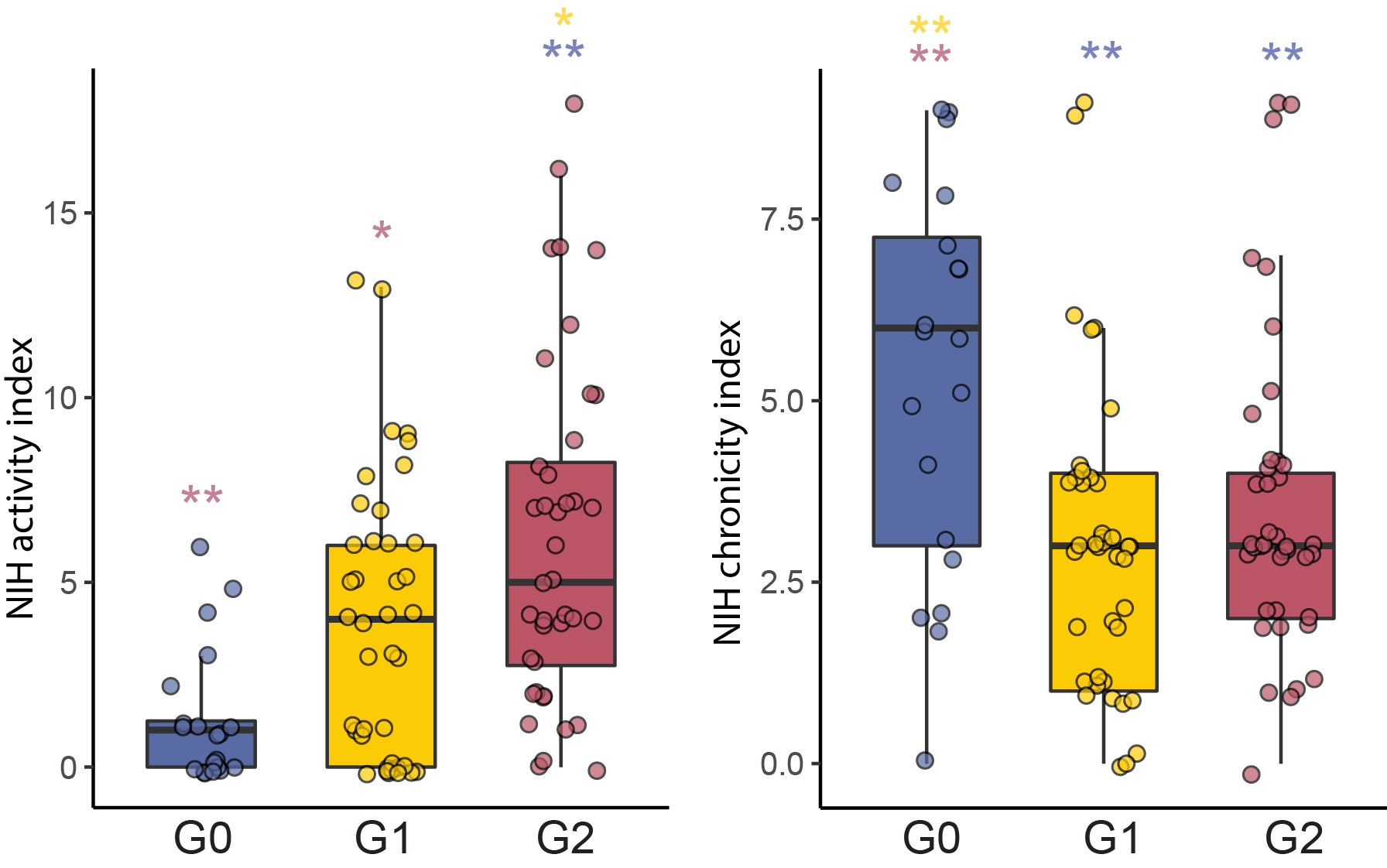
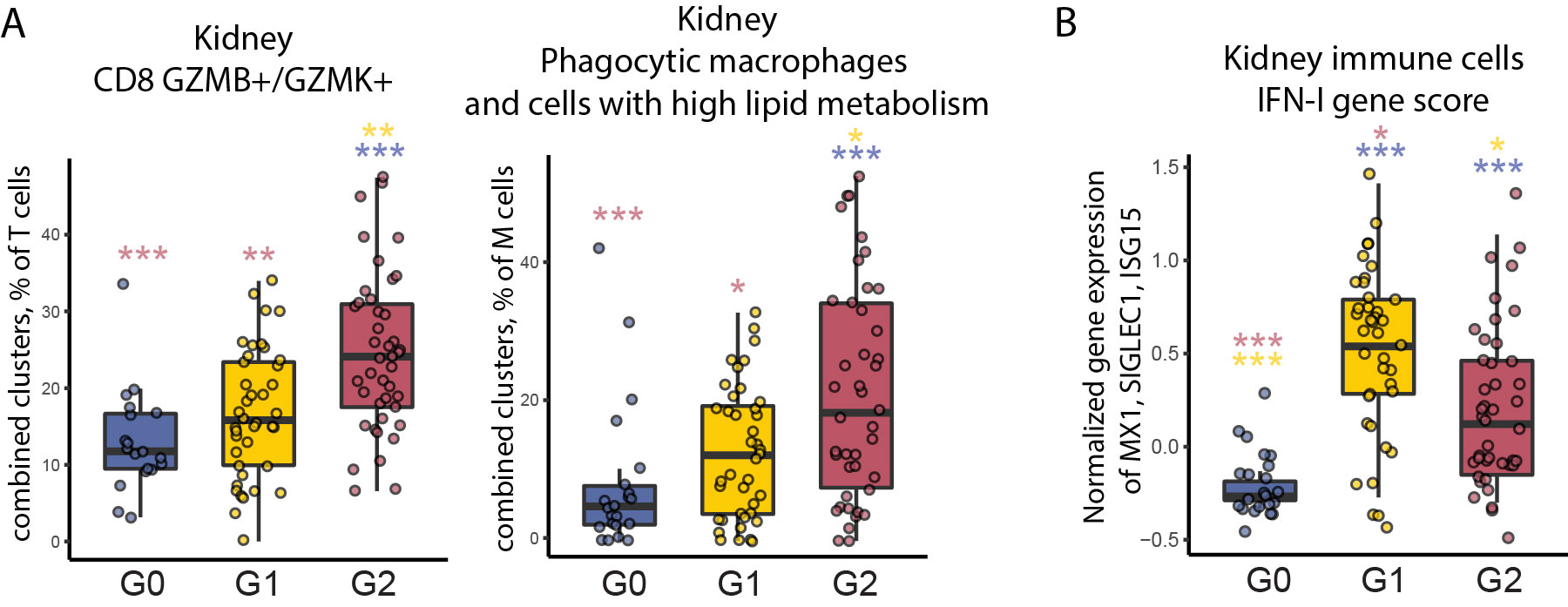
Results: Unsupervised analysis of all samples identified 3 patient subgroups based on blood immunophenotypes (Figure 1A). The first group (G0) included all controls and 20% LN patients; the two others (G1, G2) included only LN patients. A cytometric IFN-I score, based on MX1, siglec1 and ISG15 expression, was significantly different between the three groups; G0-LN patients had comparable scores to controls and G1 displayed the highest values (Figure 1B). G2 membership was driven by an increased proportion of GZMB+ GZMK+/- CD8 T cells, plus increased CD86dim monocytes and activated B cells including CD11chi cells (Figure 1C). G2 was associated with higher histologic activity scores, whereas G0 had higher chronicity scores, even after controlling for race, corticoid dose, immunosuppressant use, and previous history of renal biopsy (Figure 2). Complete renal response (CR), determined at 1 year in patients with baseline urine protein-creatinine ratio > 1, was more frequent in G2 than in G0/G1 LN patients, independently of history of previous renal biopsy (CR in G2 = 15 [41%] vs others = 9 [18%]; OR [95%CI] = 3.9 [1.3,12.5]; p.adj = 0.02). Finally, we asked whether the composition of immune cell infiltrates in the kidney, evaluated by scRNA-seq of kidney biopsies, differed between the 3 blood-defined subgroups. G2 patients had kidney T cell infiltrates enriched in GZMB+/GZMK+ CD8 T cell subsets and in myeloid cells with an activated and phagocytic profile compared to G0/G1 patients. In contrast, G1 patients showed the highest expression of IFN-I gene signature across the groups, consistent with the pattern seen in blood (Figure 3).
Conclusion: Blood immunophenotyping identified 3 groups of LN patients with different patterns of immune cell infiltration and likelihood of response to treatment. Cytometric profiles distinguished patients with a signature involving activated CD8 T cells, CD11chi B cells and activated myeloid cells (G2) from those with the highest IFN scores (G1). Patients with increased blood and kidney CD8 GZMB+GZMK+/- cells (G2) had increased renal activity scores at baseline and a higher likelihood of response at 1 year.
A. Horisberger: None; A. Griffith: None; A. Arazi: None; J. Keegan: None; K. Howard: None; T. Sasaki: None; T. Ghosh: None; A. Fava: Annexon Biosciences, 2, Sanofi, 1; J. Inamo: None; J. Pulford: None; E. Murzin: None; B. Hancock: None; K. Preisinger: None; M. Gutierrez-Arcelus: None; T. Eisenhaure: None; J. Guthridge: None; P. Hoover: None; M. Dall'Era: Annexon Biosciences, 2, 5, AstraZeneca, 2, Aurinia, 2, Biogen, 2, GlaxoSmithKlein, 2, 5, Pfizer, 2; D. Wofsy: Amgen, 7, Novartis, 7; D. Kamen: None; K. Kalunian: AbbVie/Abbott, 2, Amgen, 5, AstraZeneca, 2, Aurinia, 2, Bristol-Myers Squibb(BMS), 2, Eli Lilly, 2, EquilliumBio, 2, Genentech, 2, Gilead, 2, Janssen, 2, KezarBio, 1, Merck/MSD, 2, Novartis, 2, Pfizer, 2, Remegene, 2, Roche, 2, UCB, 5; R. Furie: Biogen, 2, 5; H. Belmont: Alexion, 6, Aurinia, 6; P. Izmirly: None; R. Clancy: None; D. Hildeman: None; s. Woodle: None; W. Apruzzese: Pfizer, 3; M. McMahon: None; J. Grossman: None; J. Barnas: None; F. Payan-Schober: None; M. Ishimori: None; A. RA SLE Network: None; M. Kretzler: amfAR, 5, Angion, 5, Astellas, 2, AstraZeneca, 5, Boehringer-Ingelheim, 5, Certa, 5, Chinook, 5, Eli Lilly, 5, Gilead, 5, Goldfinch Bio, 5, IONIS, 5, Janssen, 2, 5, Maze Therapeutics, 5, Moderna, 5, NovoNordisk, 5, Poxel, 2, Regeneron, 5, RenalytixAI, 5, Travere, 5, UCB, 2; C. Berthier: None; J. Hodgin: AstraZeneca, 5, 6, Eli Lilly, 5, Gilead, 5, Janssen, 5, Moderna, 5, Novo Nordisk, 5, Regeneron, 5; D. Demeke: None; C. Putterman: Equillium, 2, KidneyCure, 1, Progentec, 2; N. Hacohen: None; M. Brenner: 4FO Ventures, 2, GlaxoSmithKlein(GSK), 2, Mestag Therapeutics, 2, 11, Third Rock Ventures, 2; J. Anolik: None; A. Davidson: None; J. James: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 2, Novartis, 2, Progentec Biosciences, 5; S. Raychaudhuri: AbbVie, 6, Janssen, 1, Mestag, Inc, 2, 8, Pfizer, 1, Sanofi, 1, Sonoma, 1, 8; M. Petri: Alexion, 1, Amgen, 1, AnaptysBio, 1, Annexon Bio, 1, Argenx, 1, Arhros-Focus Med/Ed, 6, AstraZeneca, 1, 5, Aurinia, 1, 5, 6, Axdev, 1, Biogen, 1, Boxer Capital, 2, Cabaletto Bio, 2, Caribou Biosciences, 2, CVS Health, 1, Eli Lilly, 1, 5, Emergent Biosolutions, 1, Exagen, 5, Exo Therapeutics, 2, Gilead Biosciences, 2, GlaxoSmithKlein(GSK), 1, 5, 6, Horizon Therapeutics, 2, Idorsia Pharmaceuticals, 2, IQVIA, 1, Janssen, 1, 5, Kira Pharmaceuticals, 2, MedShr, 6, Merck/EMD Serono, 1, Momenta Pharmaceuticals, 2, Nexstone Immunology, 2, Nimbus Lakshmi, 2, Proviant, 2, Sanofi, 2, Sinomab Biosciences, 2, Thermofisher, 5, UCB, 2; J. Buyon: Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, Related Sciences, 1; B. Diamond: Alpine, 12, DSMB, DBV, 2, 2, IMT, 2, Kyverna, 2, Nighthawk, 2, ONO, 2; T. In RA/SLE: None; F. Zhang: None; J. Lederer: None; D. Rao: AstraZeneca, 2, Bristol-Myers Squibb, 2, 5, GlaxoSmithKlein(GSK), 2, Hifibio, 2, Janssen, 5, Merck, 5, Scipher Medicine, 2.
Background/Purpose: Patients with lupus nephritis (LN) have variable responses to standard-of-care therapy, and a third of patients with class III, IV, or V show a progressive decline in kidney function. Identifying distinct inflammatory processes associated with LN using non-invasive tools may improve treatment targeting. Here, we aimed to identify subgroups of LN patients that differ in systemic immune activity and to evaluate their relationship to kidney pathology.
Methods: Mass cytometry using four 48-marker panels was applied to characterize peripheral blood mononuclear cells from 140 patients with active, biopsy-proven proliferative (class III or IV +/- V, n=98) or membranous (class V, n=42) nephritis and 40 healthy controls in the Accelerated Medicine Partnership RA/SLE Network Phase II study. K-means clustering was used to stratify patients based on the proportions of 55 immune cell subsets defined using B cell-, T cell-, myeloid cell-, and NK cell-focused panels.
Results: Unsupervised analysis of all samples identified 3 patient subgroups based on blood immunophenotypes (Figure 1A). The first group (G0) included all controls and 20% LN patients; the two others (G1, G2) included only LN patients. A cytometric IFN-I score, based on MX1, siglec1 and ISG15 expression, was significantly different between the three groups; G0-LN patients had comparable scores to controls and G1 displayed the highest values (Figure 1B). G2 membership was driven by an increased proportion of GZMB+ GZMK+/- CD8 T cells, plus increased CD86dim monocytes and activated B cells including CD11chi cells (Figure 1C). G2 was associated with higher histologic activity scores, whereas G0 had higher chronicity scores, even after controlling for race, corticoid dose, immunosuppressant use, and previous history of renal biopsy (Figure 2). Complete renal response (CR), determined at 1 year in patients with baseline urine protein-creatinine ratio > 1, was more frequent in G2 than in G0/G1 LN patients, independently of history of previous renal biopsy (CR in G2 = 15 [41%] vs others = 9 [18%]; OR [95%CI] = 3.9 [1.3,12.5]; p.adj = 0.02). Finally, we asked whether the composition of immune cell infiltrates in the kidney, evaluated by scRNA-seq of kidney biopsies, differed between the 3 blood-defined subgroups. G2 patients had kidney T cell infiltrates enriched in GZMB+/GZMK+ CD8 T cell subsets and in myeloid cells with an activated and phagocytic profile compared to G0/G1 patients. In contrast, G1 patients showed the highest expression of IFN-I gene signature across the groups, consistent with the pattern seen in blood (Figure 3).
Conclusion: Blood immunophenotyping identified 3 groups of LN patients with different patterns of immune cell infiltration and likelihood of response to treatment. Cytometric profiles distinguished patients with a signature involving activated CD8 T cells, CD11chi B cells and activated myeloid cells (G2) from those with the highest IFN scores (G1). Patients with increased blood and kidney CD8 GZMB+GZMK+/- cells (G2) had increased renal activity scores at baseline and a higher likelihood of response at 1 year.



A. Horisberger: None; A. Griffith: None; A. Arazi: None; J. Keegan: None; K. Howard: None; T. Sasaki: None; T. Ghosh: None; A. Fava: Annexon Biosciences, 2, Sanofi, 1; J. Inamo: None; J. Pulford: None; E. Murzin: None; B. Hancock: None; K. Preisinger: None; M. Gutierrez-Arcelus: None; T. Eisenhaure: None; J. Guthridge: None; P. Hoover: None; M. Dall'Era: Annexon Biosciences, 2, 5, AstraZeneca, 2, Aurinia, 2, Biogen, 2, GlaxoSmithKlein, 2, 5, Pfizer, 2; D. Wofsy: Amgen, 7, Novartis, 7; D. Kamen: None; K. Kalunian: AbbVie/Abbott, 2, Amgen, 5, AstraZeneca, 2, Aurinia, 2, Bristol-Myers Squibb(BMS), 2, Eli Lilly, 2, EquilliumBio, 2, Genentech, 2, Gilead, 2, Janssen, 2, KezarBio, 1, Merck/MSD, 2, Novartis, 2, Pfizer, 2, Remegene, 2, Roche, 2, UCB, 5; R. Furie: Biogen, 2, 5; H. Belmont: Alexion, 6, Aurinia, 6; P. Izmirly: None; R. Clancy: None; D. Hildeman: None; s. Woodle: None; W. Apruzzese: Pfizer, 3; M. McMahon: None; J. Grossman: None; J. Barnas: None; F. Payan-Schober: None; M. Ishimori: None; A. RA SLE Network: None; M. Kretzler: amfAR, 5, Angion, 5, Astellas, 2, AstraZeneca, 5, Boehringer-Ingelheim, 5, Certa, 5, Chinook, 5, Eli Lilly, 5, Gilead, 5, Goldfinch Bio, 5, IONIS, 5, Janssen, 2, 5, Maze Therapeutics, 5, Moderna, 5, NovoNordisk, 5, Poxel, 2, Regeneron, 5, RenalytixAI, 5, Travere, 5, UCB, 2; C. Berthier: None; J. Hodgin: AstraZeneca, 5, 6, Eli Lilly, 5, Gilead, 5, Janssen, 5, Moderna, 5, Novo Nordisk, 5, Regeneron, 5; D. Demeke: None; C. Putterman: Equillium, 2, KidneyCure, 1, Progentec, 2; N. Hacohen: None; M. Brenner: 4FO Ventures, 2, GlaxoSmithKlein(GSK), 2, Mestag Therapeutics, 2, 11, Third Rock Ventures, 2; J. Anolik: None; A. Davidson: None; J. James: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 2, Novartis, 2, Progentec Biosciences, 5; S. Raychaudhuri: AbbVie, 6, Janssen, 1, Mestag, Inc, 2, 8, Pfizer, 1, Sanofi, 1, Sonoma, 1, 8; M. Petri: Alexion, 1, Amgen, 1, AnaptysBio, 1, Annexon Bio, 1, Argenx, 1, Arhros-Focus Med/Ed, 6, AstraZeneca, 1, 5, Aurinia, 1, 5, 6, Axdev, 1, Biogen, 1, Boxer Capital, 2, Cabaletto Bio, 2, Caribou Biosciences, 2, CVS Health, 1, Eli Lilly, 1, 5, Emergent Biosolutions, 1, Exagen, 5, Exo Therapeutics, 2, Gilead Biosciences, 2, GlaxoSmithKlein(GSK), 1, 5, 6, Horizon Therapeutics, 2, Idorsia Pharmaceuticals, 2, IQVIA, 1, Janssen, 1, 5, Kira Pharmaceuticals, 2, MedShr, 6, Merck/EMD Serono, 1, Momenta Pharmaceuticals, 2, Nexstone Immunology, 2, Nimbus Lakshmi, 2, Proviant, 2, Sanofi, 2, Sinomab Biosciences, 2, Thermofisher, 5, UCB, 2; J. Buyon: Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, Related Sciences, 1; B. Diamond: Alpine, 12, DSMB, DBV, 2, 2, IMT, 2, Kyverna, 2, Nighthawk, 2, ONO, 2; T. In RA/SLE: None; F. Zhang: None; J. Lederer: None; D. Rao: AstraZeneca, 2, Bristol-Myers Squibb, 2, 5, GlaxoSmithKlein(GSK), 2, Hifibio, 2, Janssen, 5, Merck, 5, Scipher Medicine, 2.



