Abstract Session
Reproductive health
Session: Abstracts: Reproductive Issues in Rheumatic Disorders (1681–1686)
1681: Tacrolimus Use in SLE Pregnancy and Its Effect on Pregnancy Outcomes: Retrospective Study in Two Japanese Tertiary Referral Centers
Monday, November 13, 2023
4:00 PM - 4:10 PM PT
Location: Room 25A-C

Takehiro Nakai, MD
St. Luke's International Hospital
Tokyo, JapanDisclosure information not submitted.
Presenting Author(s)
Takehiro Nakai1, Nanase Honda2, Eri Soga2, Sho Fukui3, Ayako Kitada1, Naoto Yokogawa4 and Masato Okada1, 1St. Luke's International Hospital, Tokyo, Japan, 2Tokyo Metropolitan Tama Medical Center, Tama, Japan, 3Brigham and Women's Hospital, Boston, MA, 4Tokyo Metropolitan Tama Medical Center, Taito City, Tokyo, Japan
Background/Purpose: Tacrolimus is one of the major treatment options of systemic lupus erythematosus (SLE) and is thought to be pregnancy compatible medication. Since little is known on tacrolimus use during pregnancy complicated by SLE, we conducted this study.
Methods: We included pregnant patients with SLE who were followed up at two Japanese tertiary referral centres. The pregnant patients with tacrolimus exposure and those without it were propensity score matched in a ratio of one-to-two to minimise difference in disease severity. Thereafter, we compared adverse pregnancy outcome (APO) ratio according to tacrolimus exposure.
Results: Of the 124 pregnancies, 29 were exposed to tacrolimus. The pregnant patients with tacrolimus exposure tended to suffer from lupus nephritis than did those without exposure (51.7% versus 17.9%, p< 0.01) . Moreover, they tended to be treated with hydroxychloroquine/higher dose of glucocorticoid and experienced lupus flare at conception compared with those without exposure (hydroxychloroquine: 69.9% versus 28.4%, p< 0.01; glucocorticoid dosage: 5.00 [5.00, 10.00] versus 5.00 [1.00, 7.75] mg/day, p=0.03; lupus flare: 19.2% versus 1.4%, p< 0.01).
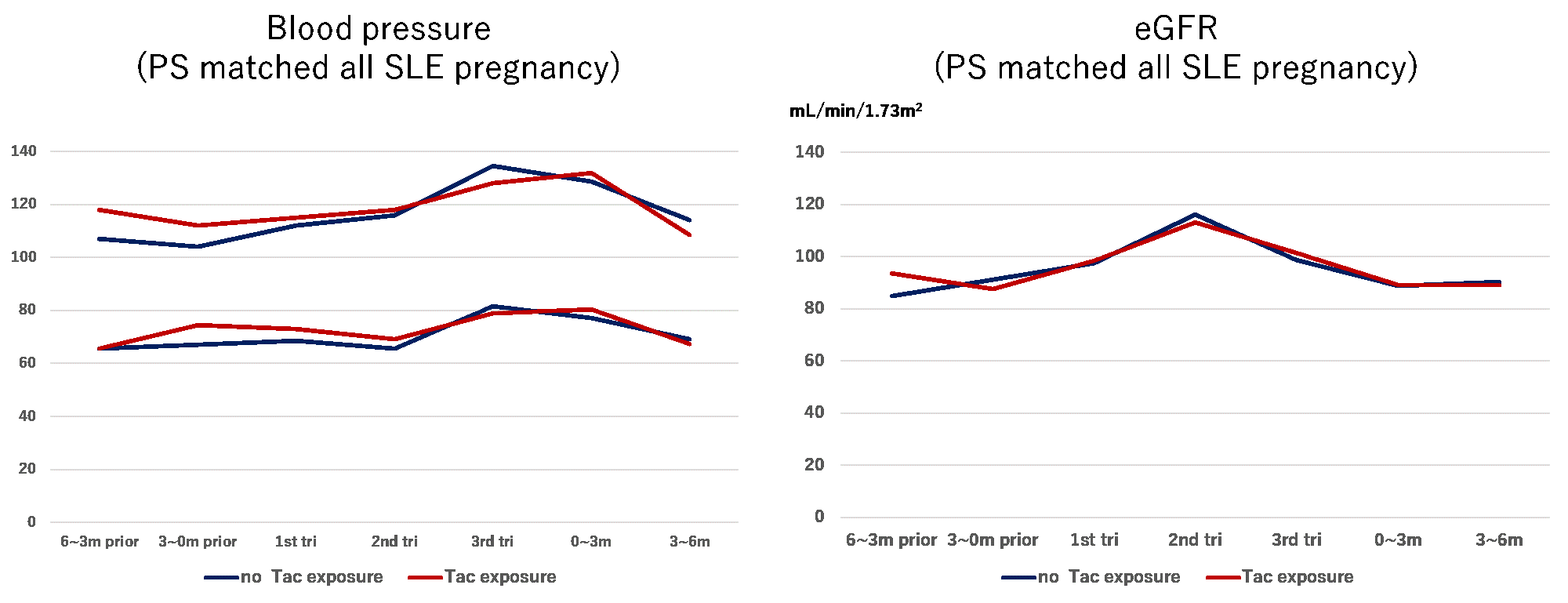
After propensity score matching, group difference in the baseline characteristics including SLE severity diminished. In addition, no statistical differences were also noted in the APO ratio; blood pressure; and estimated glomerular filtration rate during pregnancy and after delivery between the groups. (overall APO: 47.1% versus 47.1%, p=1.0; maternal APO: 29.4% versus 26.5%, p=1.0; neonatal APO: 58.8% versus 41.2%, p=0.25; PROMISSE APO: 17.6% versus 17.6%, p=1.0; hypertensive disorders of pregnancy: 17.6% versus 14.7%, p=1.0; preeclampsia: 5.9% versus 5.9%, p=1.0)
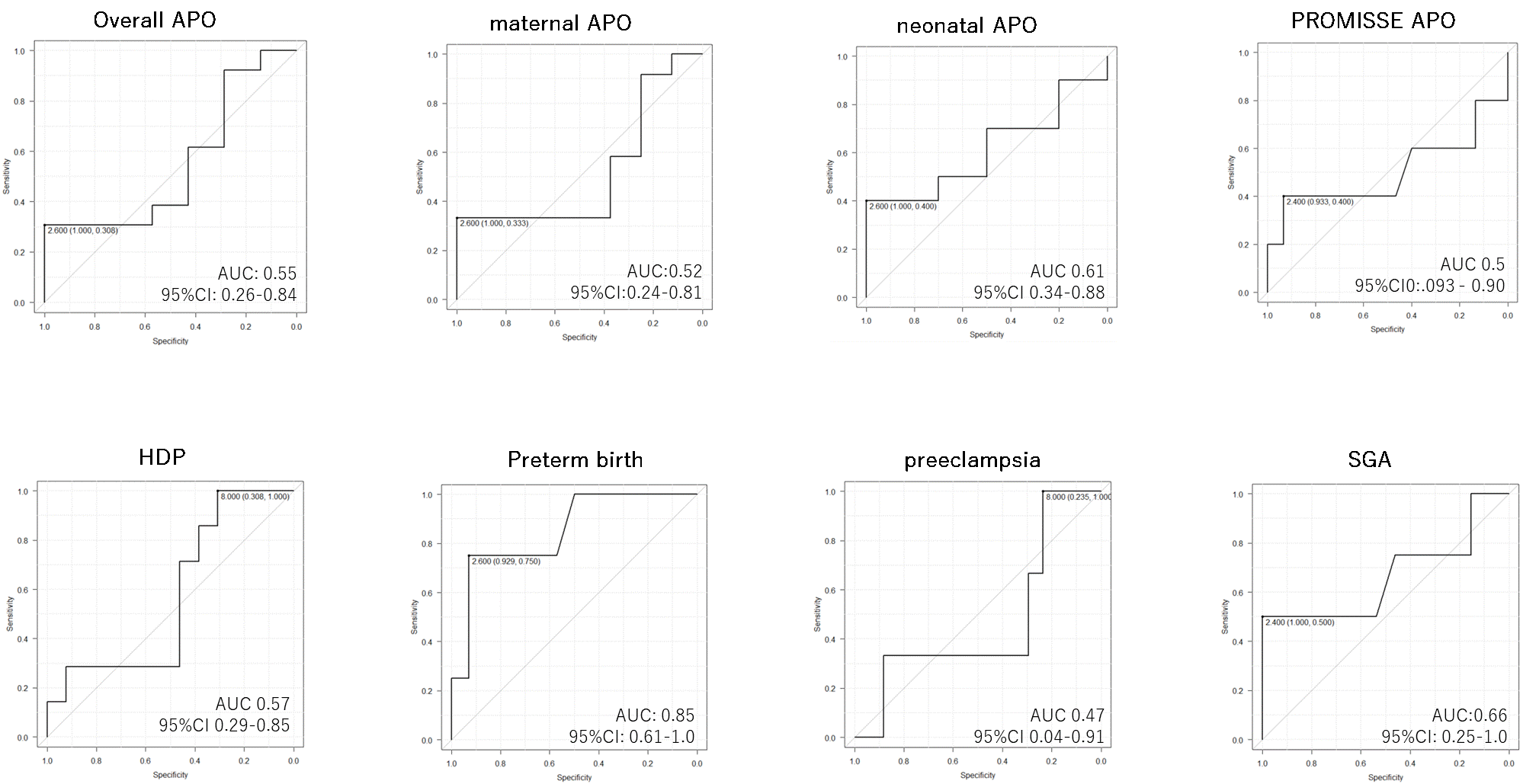
Furthermore, receiver operating characteristic curve showed that tacrolimus concentration >2.6ng/ml was related to reduced preterm birth rate.
Conclusion: Tacrolimus use during pregnancy showed no significant impact on APO ratio, blood pressure, or renal function. Therefore, tacrolimus use might be acceptable to control lupus activity during pregnancy.
In addition, when administering tacrolimus during pregnancy, it is advisable to maintain its concentration ≥2.6 ng/ml in order to reduce the risk for preterm birth while paying careful attention to possible maternal side effects of tacrolimus.
.gif)
T. Nakai: None; N. Honda: None; E. Soga: None; S. Fukui: None; A. Kitada: None; N. Yokogawa: None; M. Okada: AbbVie/Abbott, 6, Eli Lilly, 6, Pfizer, 6.
Background/Purpose: Tacrolimus is one of the major treatment options of systemic lupus erythematosus (SLE) and is thought to be pregnancy compatible medication. Since little is known on tacrolimus use during pregnancy complicated by SLE, we conducted this study.
Methods: We included pregnant patients with SLE who were followed up at two Japanese tertiary referral centres. The pregnant patients with tacrolimus exposure and those without it were propensity score matched in a ratio of one-to-two to minimise difference in disease severity. Thereafter, we compared adverse pregnancy outcome (APO) ratio according to tacrolimus exposure.
Results: Of the 124 pregnancies, 29 were exposed to tacrolimus. The pregnant patients with tacrolimus exposure tended to suffer from lupus nephritis than did those without exposure (51.7% versus 17.9%, p< 0.01) . Moreover, they tended to be treated with hydroxychloroquine/higher dose of glucocorticoid and experienced lupus flare at conception compared with those without exposure (hydroxychloroquine: 69.9% versus 28.4%, p< 0.01; glucocorticoid dosage: 5.00 [5.00, 10.00] versus 5.00 [1.00, 7.75] mg/day, p=0.03; lupus flare: 19.2% versus 1.4%, p< 0.01).
After propensity score matching, group difference in the baseline characteristics including SLE severity diminished. In addition, no statistical differences were also noted in the APO ratio; blood pressure; and estimated glomerular filtration rate during pregnancy and after delivery between the groups. (overall APO: 47.1% versus 47.1%, p=1.0; maternal APO: 29.4% versus 26.5%, p=1.0; neonatal APO: 58.8% versus 41.2%, p=0.25; PROMISSE APO: 17.6% versus 17.6%, p=1.0; hypertensive disorders of pregnancy: 17.6% versus 14.7%, p=1.0; preeclampsia: 5.9% versus 5.9%, p=1.0)
Furthermore, receiver operating characteristic curve showed that tacrolimus concentration >2.6ng/ml was related to reduced preterm birth rate.
Conclusion: Tacrolimus use during pregnancy showed no significant impact on APO ratio, blood pressure, or renal function. Therefore, tacrolimus use might be acceptable to control lupus activity during pregnancy.
In addition, when administering tacrolimus during pregnancy, it is advisable to maintain its concentration ≥2.6 ng/ml in order to reduce the risk for preterm birth while paying careful attention to possible maternal side effects of tacrolimus.
.gif)
Table 1: prevalence of adverse pregnancy outcome according to the use of tacrolimus

Figure 1: change in the blood pressure before and during pregnancy and after delivery

Figure 2 ROC curve for the maximum tacrolimus concentration during pregnancy and each adverse pregnancy outcome
T. Nakai: None; N. Honda: None; E. Soga: None; S. Fukui: None; A. Kitada: None; N. Yokogawa: None; M. Okada: AbbVie/Abbott, 6, Eli Lilly, 6, Pfizer, 6.



