Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Etiology & Pathogenesis (1597–1602)
1601: Interleukin-6 Disrupts Blood-cerebrospinal Fluid Barrier Permeability in Murine Neuropsychiatric Lupus
Monday, November 13, 2023
3:00 PM - 3:10 PM PT
Location: Room 6F/6C
- CP
Chaim Putterman, MD
Albert Einstein College of Medicine
Bronx, NY, United StatesDisclosure information not submitted.
Presenting Author(s)
Joshua Reynolds1, Lola Torz2, Natalia Petersen2, Ayal Ben-Zvi3 and Chaim Putterman1, 1Albert Einstein College of Medicine, Bronx, NY, 2Novo Nordic, Global Obesity Research, Malov, Denmark, 3Hebrew University of Jerusalem, Jerusalem, Israel
Background/Purpose: In exploring the pathogenic mechanisms underlying neuropsychiatric lupus (NPSLE), it was discovered that cerebrospinal fluid (CSF) from lupus patients often contains neurotoxic antibodies, cytokines, and metabolites. Disruption of the blood-CSF barrier (B-CSFB), formed by choroid plexus (CP) epithelia, could explain these abnormalities. In both NPSLE patients and lupus mouse models, immune cells infiltrate the CP and can alter normal epithelial functions through extensive cytokine signaling. Interleukin-6 (IL-6) is elevated in the serum and CSF of both NPSLE patients and lupus mice. ATP-binding cassette (ABC) transporter function in the CP, including P-gp, MRP1, and BCRP, is fundamental to the clearance of neurotoxic substrates from the CSF. In this study, we assessed the effects of IL-6 on lupus mouse derived CP organoids to investigate the integrity of the B-CSFB in NPSLE.
Methods: CP tissue from 2-5 female MRL/lpr (WT) or IL-6 knockout (KO) mice of at least 16 weeks of age were pooled and cultured for two weeks to generate CP organoids for each experiment, as described (Petersen et al, 2020). Organoid morphology was confirmed using whole-mount immunostaining and electron microscopy. Gene expression was measured by real-time qPCR. A standard time-lapse permeability assay which quantified tracer fluorescence within each organoid's central vacuole was performed under various conditions. We assessed organoid permeability to tracer in the presence of supplemental IL-6 (10 ng/mL) or anti-IL-6 receptor antibodies (1 ug/mL). We also assessed inherent tracer permeability differences between IL-6 competent WT and IL-6 KO MRL/lpr-derived organoids. The fluorescent tracers used included two small dyes, lucifer yellow (LY) and sodium fluorescein (SF), and a larger 10 kDa dextran molecule (Dex). Importantly, LY but not SF is transported by ABC transporters. Two-tail Mann-Whitney u tests compared differences between groups.
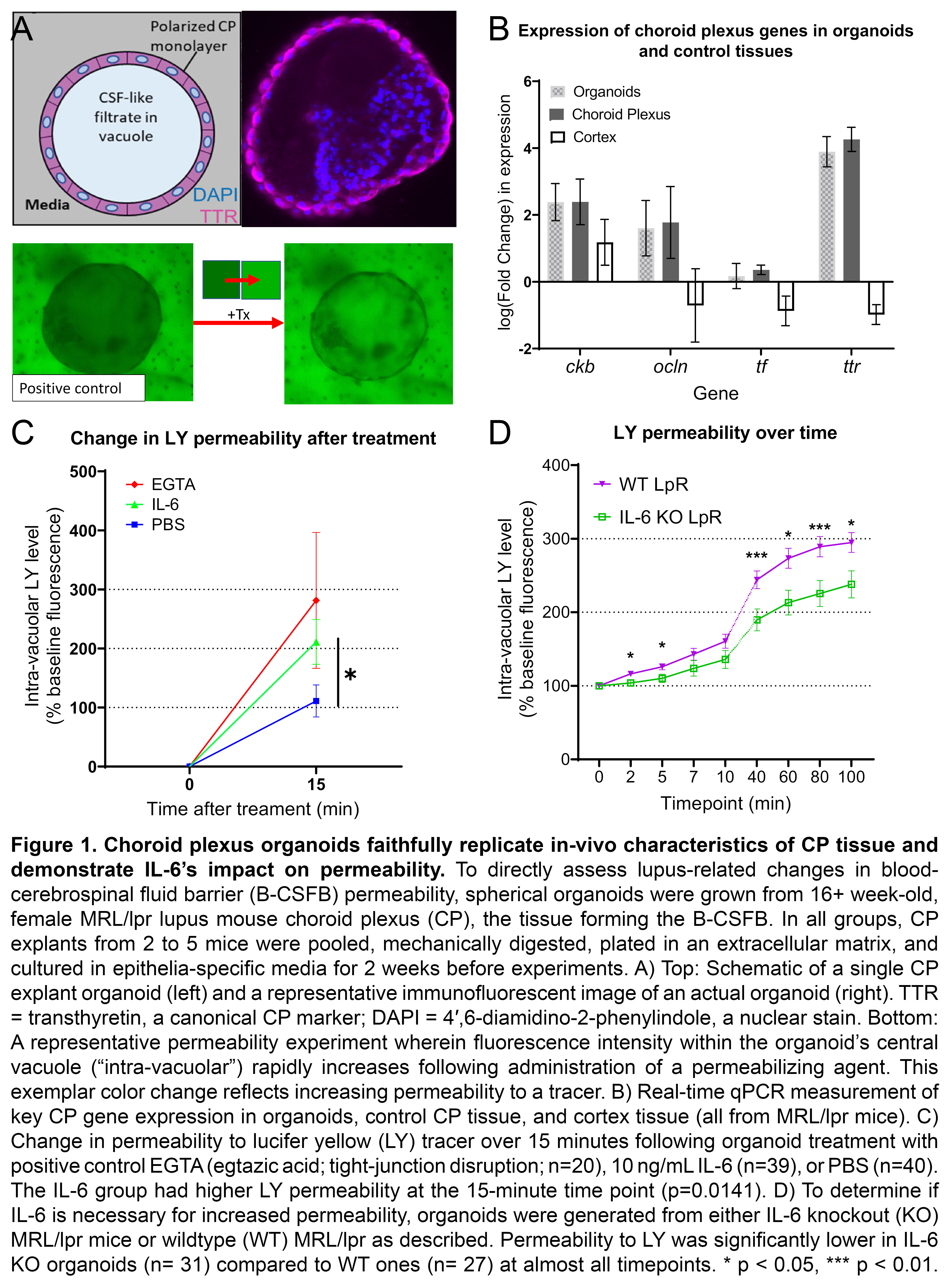
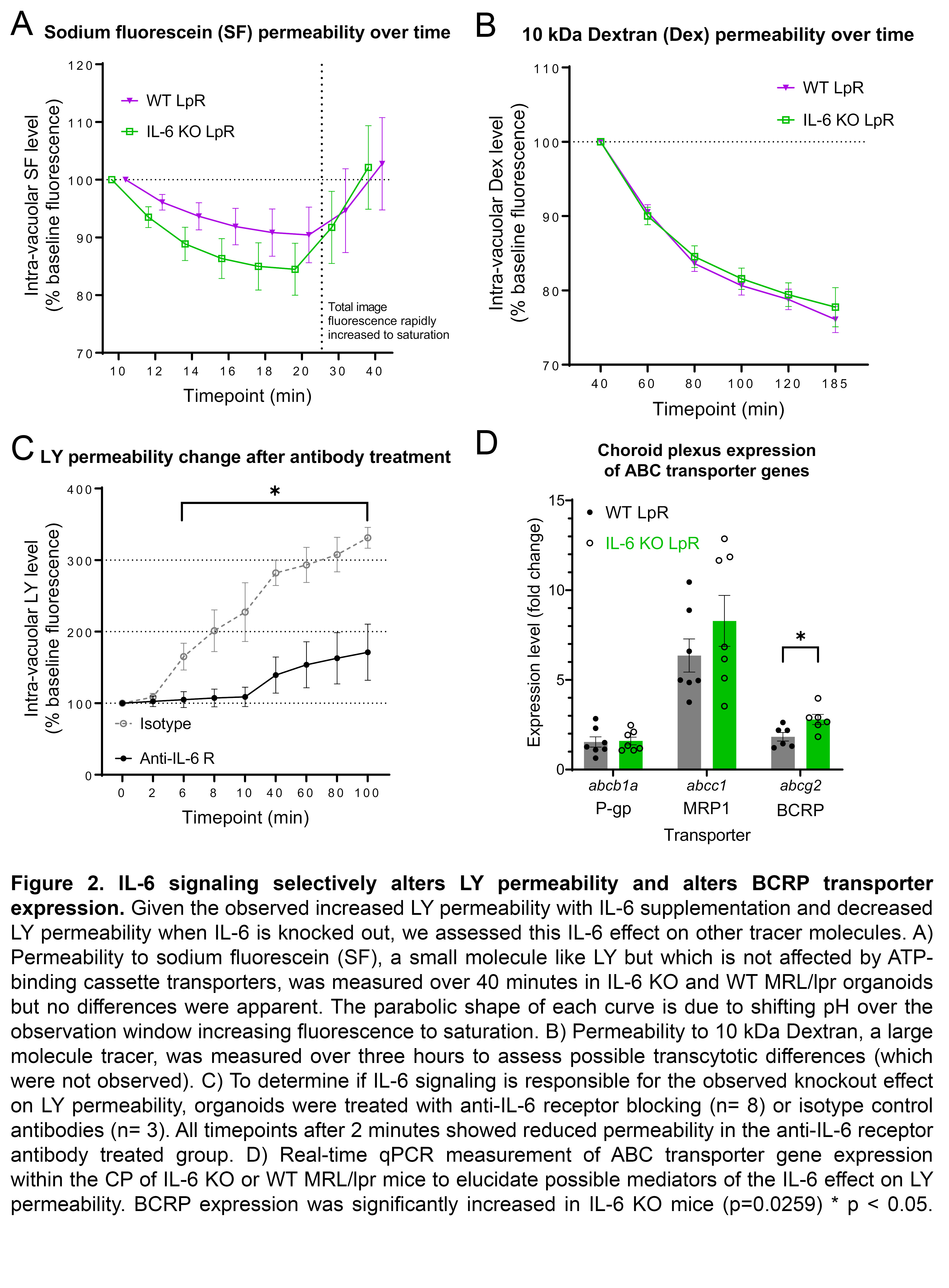
Results: MRL/lpr-derived organoids replicated the in-vivo morphology and gene expression of the CP (Fig 1A-B). IL-6 rapidly increased MRL/lpr organoid permeability to LY (IL-6: n=39, 174.4+/- 37.91; PBS: n=40, 76.1+/-27.1; p=0.014; Fig 1C). IL-6 KO organoids demonstrated consistently reduced permeability to LY compared to WT (KO: n=31; WT: n=27; p< < 0.05; Fig 1D). No permeability differences between IL-6 KO (n=19) and WT (n=19) organoids were seen for SF (Fig 2A) or Dex (Fig 2B). Incubation with an anti-IL-6 receptor antibody (n=8) significantly decreased LY permeability relative to an isotype control (n=3; Fig 2C). In-vivo expression of abcg2, a constituent of BCRP, was increased in IL-6 KO MRL/lpr mice (KO: n=6, 8.83+/-0.84; WT: n=4.17+/-0.61; p=0.026; Fig 2D).
Conclusion: In the first application of a novel CP organoid model to study lupus, we found that IL-6 signaling appears sufficient and necessary to increase permeability to LY, but not other tracers studied. Changes in BCRP, which effluxes only LY, could explain this accumulation of LY within organoids. Therefore, the high IL-6 environment of SLE could interfere with BCRP function and hinder the CP's ability to clear potentially neurotoxic metabolites, and thus contribute to the pathogenesis of NPSLE.
J. Reynolds: None; L. Torz: None; N. Petersen: None; A. Ben-Zvi: None; C. Putterman: Equillium, 2, KidneyCure, 1, Progentec, 2.
Background/Purpose: In exploring the pathogenic mechanisms underlying neuropsychiatric lupus (NPSLE), it was discovered that cerebrospinal fluid (CSF) from lupus patients often contains neurotoxic antibodies, cytokines, and metabolites. Disruption of the blood-CSF barrier (B-CSFB), formed by choroid plexus (CP) epithelia, could explain these abnormalities. In both NPSLE patients and lupus mouse models, immune cells infiltrate the CP and can alter normal epithelial functions through extensive cytokine signaling. Interleukin-6 (IL-6) is elevated in the serum and CSF of both NPSLE patients and lupus mice. ATP-binding cassette (ABC) transporter function in the CP, including P-gp, MRP1, and BCRP, is fundamental to the clearance of neurotoxic substrates from the CSF. In this study, we assessed the effects of IL-6 on lupus mouse derived CP organoids to investigate the integrity of the B-CSFB in NPSLE.
Methods: CP tissue from 2-5 female MRL/lpr (WT) or IL-6 knockout (KO) mice of at least 16 weeks of age were pooled and cultured for two weeks to generate CP organoids for each experiment, as described (Petersen et al, 2020). Organoid morphology was confirmed using whole-mount immunostaining and electron microscopy. Gene expression was measured by real-time qPCR. A standard time-lapse permeability assay which quantified tracer fluorescence within each organoid's central vacuole was performed under various conditions. We assessed organoid permeability to tracer in the presence of supplemental IL-6 (10 ng/mL) or anti-IL-6 receptor antibodies (1 ug/mL). We also assessed inherent tracer permeability differences between IL-6 competent WT and IL-6 KO MRL/lpr-derived organoids. The fluorescent tracers used included two small dyes, lucifer yellow (LY) and sodium fluorescein (SF), and a larger 10 kDa dextran molecule (Dex). Importantly, LY but not SF is transported by ABC transporters. Two-tail Mann-Whitney u tests compared differences between groups.
Results: MRL/lpr-derived organoids replicated the in-vivo morphology and gene expression of the CP (Fig 1A-B). IL-6 rapidly increased MRL/lpr organoid permeability to LY (IL-6: n=39, 174.4+/- 37.91; PBS: n=40, 76.1+/-27.1; p=0.014; Fig 1C). IL-6 KO organoids demonstrated consistently reduced permeability to LY compared to WT (KO: n=31; WT: n=27; p< < 0.05; Fig 1D). No permeability differences between IL-6 KO (n=19) and WT (n=19) organoids were seen for SF (Fig 2A) or Dex (Fig 2B). Incubation with an anti-IL-6 receptor antibody (n=8) significantly decreased LY permeability relative to an isotype control (n=3; Fig 2C). In-vivo expression of abcg2, a constituent of BCRP, was increased in IL-6 KO MRL/lpr mice (KO: n=6, 8.83+/-0.84; WT: n=4.17+/-0.61; p=0.026; Fig 2D).
Conclusion: In the first application of a novel CP organoid model to study lupus, we found that IL-6 signaling appears sufficient and necessary to increase permeability to LY, but not other tracers studied. Changes in BCRP, which effluxes only LY, could explain this accumulation of LY within organoids. Therefore, the high IL-6 environment of SLE could interfere with BCRP function and hinder the CP's ability to clear potentially neurotoxic metabolites, and thus contribute to the pathogenesis of NPSLE.

Figure 1. Choroid plexus organoids faithfully replicate in-vivo characteristics of CP tissue and demonstrate IL-6’s impact on permeability. To directly assess lupus-related changes in blood-cerebrospinal fluid barrier (B-CSFB) permeability, spherical organoids were grown from 16+ week-old, female MRL/lpr lupus mouse choroid plexus (CP), the tissue forming the B-CSFB. In all groups, CP explants from 2 to 5 mice were pooled, mechanically digested, plated in an extracellular matrix, and cultured in epithelia-specific media for 2 weeks before experiments. A) Top: Schematic of a single CP explant organoid (left) and a representative immunofluorescent image of an actual organoid (right). TTR = transthyretin, a canonical CP marker; DAPI = 4′,6-diamidino-2-phenylindole, a nuclear stain. Bottom: A representative permeability experiment wherein fluorescence intensity within the organoid’s central vacuole (“intra-vacuolar”) rapidly increases following administration of a permeabilizing agent. This exemplar color change reflects increasing permeability to a tracer. B) Real-time qPCR measurement of key CP gene expression in organoids, control CP tissue, and cortex tissue (all from MRL/lpr mice). C) Change in permeability to lucifer yellow (LY) tracer over 15 minutes following organoid treatment with positive control EGTA (egtazic acid; tight-junction disruption; n=20), 10 ng/mL IL-6 (n=39), or PBS (n=40). The IL-6 group had higher LY permeability at the 15-minute time point (p=0.0141). D) To determine if IL-6 is necessary for increased permeability, organoids were generated from either IL-6 knockout (KO) MRL/lpr mice or wildtype (WT) MRL/lpr as described. Permeability to LY was significantly lower in IL-6 KO organoids (n= 31) compared to WT ones (n= 27) at almost all timepoints. * p < 0.05, *** p < 0.01.

Figure 2. IL-6 signaling selectively alters LY permeability and alters BCRP transporter expression. Given the observed increased LY permeability with IL-6 supplementation and decreased LY permeability when IL-6 is knocked out, we assessed this IL-6 effect on other tracer molecules. A) Permeability to sodium fluorescein (SF), a small molecule like LY but which is not affected by ATP-binding cassette transporters, was measured over 40 minutes in IL-6 KO and WT MRL/lpr organoids but no differences were apparent. The parabolic shape of each curve is due to shifting pH over the observation window increasing fluorescence to saturation. B) Permeability to 10 kDa Dextran, a large molecule tracer, was measured over three hours to assess possible transcytotic differences (which were not observed). C) To determine if IL-6 signaling is responsible for the observed knockout effect on LY permeability, organoids were treated with anti-IL-6 receptor blocking (n= 8) or isotype control antibodies (n= 3). All timepoints after 2 minutes showed reduced permeability in the anti-IL-6 receptor antibody treated group. D) Real-time qPCR measurement of ABC transporter gene expression within the CP of IL-6 KO or WT MRL/lpr mice to elucidate possible mediators of the IL-6 effect on LY permeability. BCRP expression was significantly increased in IL-6 KO mice (p=0.0259) * p < 0.05.
J. Reynolds: None; L. Torz: None; N. Petersen: None; A. Ben-Zvi: None; C. Putterman: Equillium, 2, KidneyCure, 1, Progentec, 2.



