Abstract Session
Osteoarthritis (OA) and related disorders
Session: Abstracts: Osteoarthritis & Joint Biology – Basic Science (1591–1596)
1591: Complement Factor D/Adipsin Knockout Mice Demonstrate Disparate Pain and Structural Damage Phenotypes in Obesity-induced Post Traumatic Osteoarthritis
Monday, November 13, 2023
2:00 PM - 2:10 PM PT
Location: Room 30D-E
- KC
Kelsey Collins, PhD
UCSF
San Francisco, CA, United StatesDisclosure(s): No financial relationships with ineligible companies to disclose
Presenting Author(s)
Kelsey Collins1, Kristin Lenz2, Arin Oestreich2, Luke Springer2, Antonina Akk2, Huimin Yan2, Xiaobo Wu2, John Atkinson3, Christine Pham4 and Farshid Guilak4, 1University of California San Francisco, San Francisco, CA, 2Washington University in St. Louis, St. Louis, MO, 3Washington University School of Medicine, St. Louis, MO, 4Washington University, St. Louis, MO
Background/Purpose: Osteoarthritis (OA) is the leading cause of musculoskeletal pain, which is a primary driver of care-seeking behavior. There is lack of mechanistic information detailing the interface between OA pain and structural damage. Understanding pain signals independent of structural damage has been difficult using existing pre-clinical models. We have identified a mouse model that displays discordant knee pain and structural damage, which provides a unique opportunity to explore pain independently of structural changes. Mice that lack fat-derived adipsin (also known as complement factor D, FD) are protected from cartilage damage induced by the destabilization of the medial meniscus (DMM) but demonstrate increased sensitivity to pressure-pain hyperalgesia. The goal of this study is to determine the mechanism by which FD modulates pain in OA.
Methods: FD KO mice and WT controls (7-12/group) were fed chow (10% kcal fat) or high-fat diet (HFD, 60% kcal fat). To restore circulating FD, FD KO mice were transplanted with mouse embryonic fibroblast (MEF)-derived fat implants. At 16-weeks, mice underwent destabilization of the medial meniscus (DMM) unilaterally to induce OA. At 26-weeks, forelimb grip strength, knee hyperalgesia (via small animal algometer) and tactile allodynia (Electronic von Frey) were assessed. Knee joints were evaluated using a Modified Mankin Score and immunohistochemistry for C3 and C5-9. Bulk RNA sequencing was performed on L3-L5 dorsal root ganglia (DRG). Data were analyzed by repeated measures of ANOVA (genotype, limb, diet) and post hoc testing.
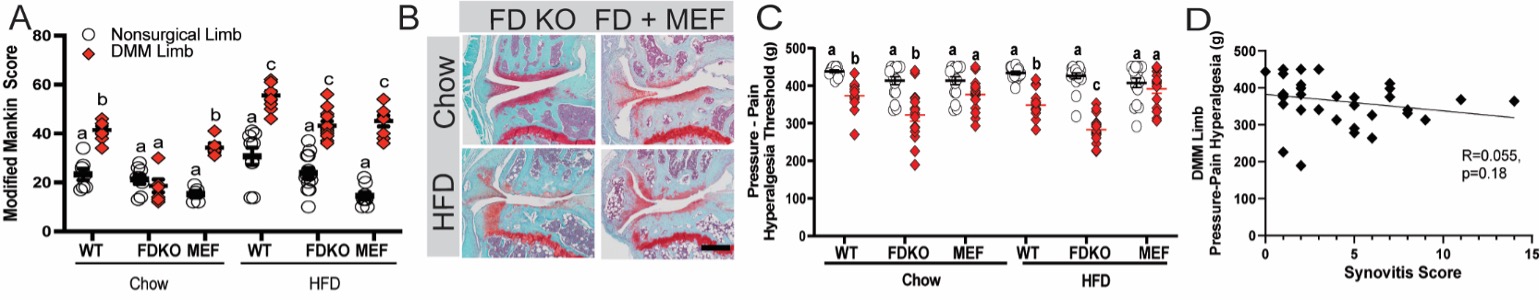
Results: Chow-fed FD KO mice were protected from DMM-induced structural OA (Fig. 1a,b) but not from increased synovitis, osteophyte formation, or pain in the DMM limb (Fig. 1c). Specifically, chow-fed FD KO mice demonstrated reduced pressure-pain thresholds and increased tactile allodynia when compared to WT DMM limb. Protection against DMM-induced cartilage damage was reversed in HFD FD KO mice (Fig.1a). There was no evidence of an alternative pathway (AP) bypass mechanism to explain this reversal in phenotype suggesting that complement is dispensable for structural damage under HFD conditions. However, HFD FD KO mice demonstrated lower pressure-pain threshold, or increased hyperalgesia at the knee (Fig. 1c), despite having a similar Modified Mankin Score to HFD WT DMM limbs (Fig. 1a,b). Restoration of AP activity using MEF transplantation in both diet groups induced a reversal of pain thresholds in FD KO to corresponding WT DMM limb levels, suggesting a role for FD in joint pain (Fig. 1c). Gene ontology (GO) analysis of DRG from FD KO mice revealed differentially expressed genes associated with neutrophil infiltration and histone modifications. Lastly, no significant relationship was found between pain and synovitis (Fig. 1d).
Conclusion: We observed paradoxically heightened pain phenotype in the FD KO mice post DMM suggesting that this model can be used to dissect the clinical discordance between subjective pain and objective joint structural damage. Understanding the mechanism by which FD modulates the DRG neuroimmune profile in regulating OA pain may inform whether therapeutic targeting of complement activity will be beneficial in OA treatment.
K. Collins: None; K. Lenz: None; A. Oestreich: Agathos Biologics, 10; L. Springer: None; A. Akk: None; H. Yan: None; X. Wu: None; J. Atkinson: Alexion Pharmaceuticals, 2, Alnylam Pharmaceuticals, 2, Celldex Therapeutics, 2, Genentech, 2, Idera Pharmaceuticals, 2, Kereos Inc, 2; C. Pham: Insmed, 1, 9; F. Guilak: None.
Background/Purpose: Osteoarthritis (OA) is the leading cause of musculoskeletal pain, which is a primary driver of care-seeking behavior. There is lack of mechanistic information detailing the interface between OA pain and structural damage. Understanding pain signals independent of structural damage has been difficult using existing pre-clinical models. We have identified a mouse model that displays discordant knee pain and structural damage, which provides a unique opportunity to explore pain independently of structural changes. Mice that lack fat-derived adipsin (also known as complement factor D, FD) are protected from cartilage damage induced by the destabilization of the medial meniscus (DMM) but demonstrate increased sensitivity to pressure-pain hyperalgesia. The goal of this study is to determine the mechanism by which FD modulates pain in OA.
Methods: FD KO mice and WT controls (7-12/group) were fed chow (10% kcal fat) or high-fat diet (HFD, 60% kcal fat). To restore circulating FD, FD KO mice were transplanted with mouse embryonic fibroblast (MEF)-derived fat implants. At 16-weeks, mice underwent destabilization of the medial meniscus (DMM) unilaterally to induce OA. At 26-weeks, forelimb grip strength, knee hyperalgesia (via small animal algometer) and tactile allodynia (Electronic von Frey) were assessed. Knee joints were evaluated using a Modified Mankin Score and immunohistochemistry for C3 and C5-9. Bulk RNA sequencing was performed on L3-L5 dorsal root ganglia (DRG). Data were analyzed by repeated measures of ANOVA (genotype, limb, diet) and post hoc testing.
Results: Chow-fed FD KO mice were protected from DMM-induced structural OA (Fig. 1a,b) but not from increased synovitis, osteophyte formation, or pain in the DMM limb (Fig. 1c). Specifically, chow-fed FD KO mice demonstrated reduced pressure-pain thresholds and increased tactile allodynia when compared to WT DMM limb. Protection against DMM-induced cartilage damage was reversed in HFD FD KO mice (Fig.1a). There was no evidence of an alternative pathway (AP) bypass mechanism to explain this reversal in phenotype suggesting that complement is dispensable for structural damage under HFD conditions. However, HFD FD KO mice demonstrated lower pressure-pain threshold, or increased hyperalgesia at the knee (Fig. 1c), despite having a similar Modified Mankin Score to HFD WT DMM limbs (Fig. 1a,b). Restoration of AP activity using MEF transplantation in both diet groups induced a reversal of pain thresholds in FD KO to corresponding WT DMM limb levels, suggesting a role for FD in joint pain (Fig. 1c). Gene ontology (GO) analysis of DRG from FD KO mice revealed differentially expressed genes associated with neutrophil infiltration and histone modifications. Lastly, no significant relationship was found between pain and synovitis (Fig. 1d).
Conclusion: We observed paradoxically heightened pain phenotype in the FD KO mice post DMM suggesting that this model can be used to dissect the clinical discordance between subjective pain and objective joint structural damage. Understanding the mechanism by which FD modulates the DRG neuroimmune profile in regulating OA pain may inform whether therapeutic targeting of complement activity will be beneficial in OA treatment.

Figure 1. Modified Mankin Score (A), red diamonds indicate limbs challenged with destabilization of the medial meniscus (DMM), circles contralateral limbs, Mouse Embryonic Fibroblast (MEF) indicates FD KO + MEF; (B) Medial tibial plateau Safranin-O/Fast Green histology sections of DMM limbs (black bar indicates 100μm); (C) pressure-pain hyperalgesia measured by SMALGO on DMM (red diamonds) and nonsurgical (circles) limbs; (D) relationship between synovitis score and DMM-limb pressure-pain hyperalgesia is not significant (p=0.18), indicating that the hyperalgesia phenotype is not explained by synovitis. Different letters indicate p<0.05 by ANOVA, data are shown as mean ± standard error.
K. Collins: None; K. Lenz: None; A. Oestreich: Agathos Biologics, 10; L. Springer: None; A. Akk: None; H. Yan: None; X. Wu: None; J. Atkinson: Alexion Pharmaceuticals, 2, Alnylam Pharmaceuticals, 2, Celldex Therapeutics, 2, Genentech, 2, Idera Pharmaceuticals, 2, Kereos Inc, 2; C. Pham: Insmed, 1, 9; F. Guilak: None.



