Abstract Session
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Diagnosis, Manifestations, & Outcomes I: AxSpA (0841–0846)
0846: Preosteoclast Plays a Pathogenic Role in Syndesmophyte Formation of Ankylosing Spondylitis Through the Secreted PDGFB - GRB2/ERK/RUNX2 Pathway
Sunday, November 12, 2023
5:15 PM - 5:25 PM PT
Location: Ballroom 20A
- YT
Presenting Author(s)
Yulong Tang1, Qingmei Liu1, Kunhai Tang1, Chengchun Geng1, Jiangnan Xie1, Jiucun Wang2, Qi Zhu3 and Jing Liu1, 1Fudan University, Shanghai, China, 2Shanghai, Shanghai, China, 3Guanghua Integrative Medicine Hospital, Shanghai, China
Background/Purpose: Ankylosing spondylitis (AS) is a chronic inflammatory disease that mainly affects the sacroiliac joint and spine. However, the real mechanisms of immune cells acting on syndesmophyte formation in AS are not well identified. We aimed to find the key AS-associated cytokine and assess its pathogenic role in AS.
Methods: A protein array with 1000 cytokines was performed in five AS patients with the first diagnosis and five age- and gender-matched healthy controls to discover the differentially expressed cytokines. The candidate differentially expressed cytokines were further quantified by multiplex protein quantitation (3 AS-associated cytokines and 3 PDGF-pathway cytokines) and ELISA (PDGFB) in independent samples (a total of 140 AS patients vs 140 healthy controls). The effects of PDGFB, the candidate cytokine, were examined by using adipose-derived stem cells (ADSCs) and human fetal osteoblast cell line (hFOB1.19) as in vitro mesenchymal cell and preosteoblast models, respectively. Furthermore, whole-transcriptome sequencing and enrichment of phosphorylated peptides were performed by using cell models to explore the underlying mechanisms of PDGFB. The xCELLigence system was applied to examine the proliferation, chemotaxis, and migration abilities of PDGFB-stimulated or -unstimulated cells.
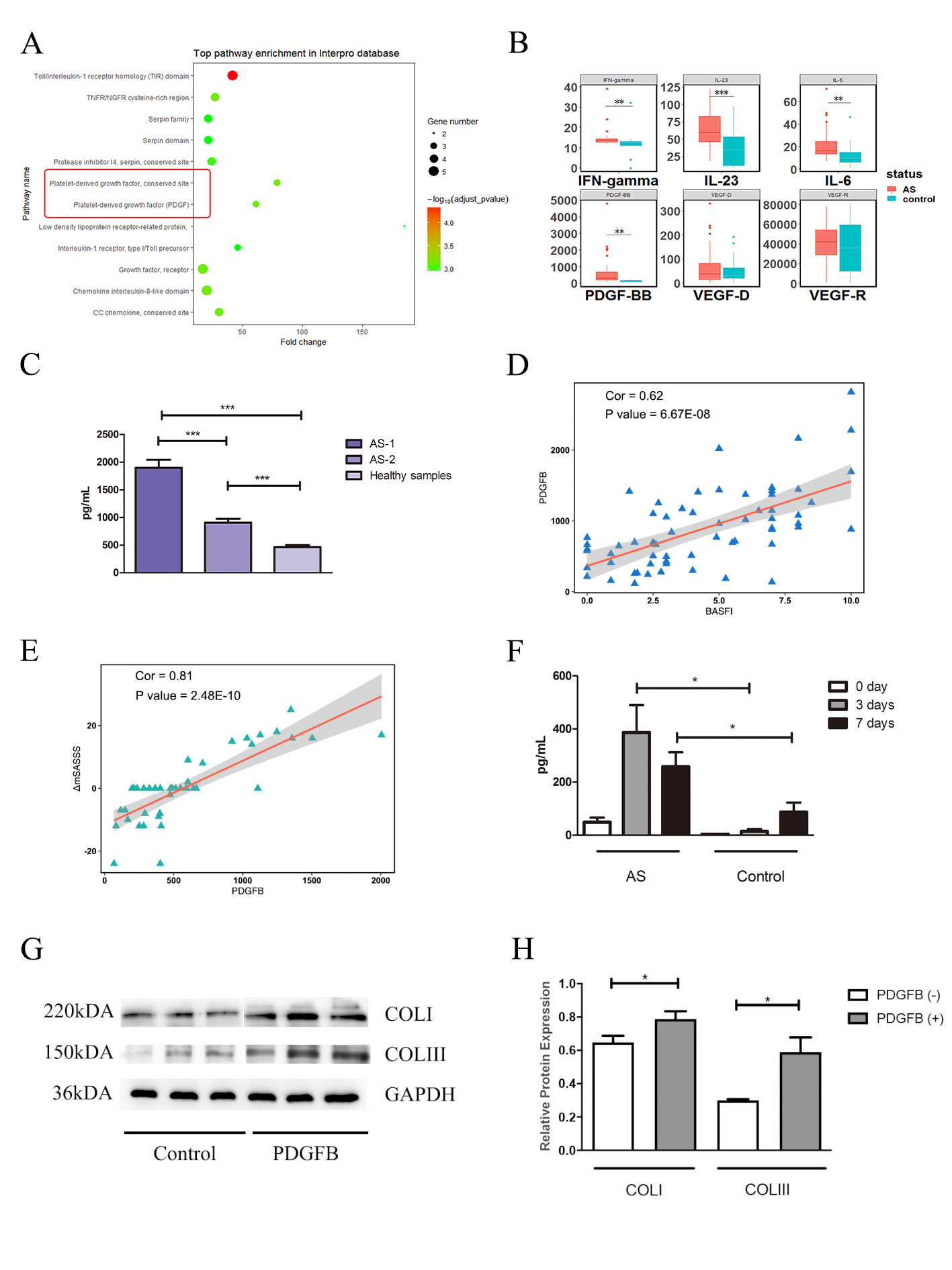
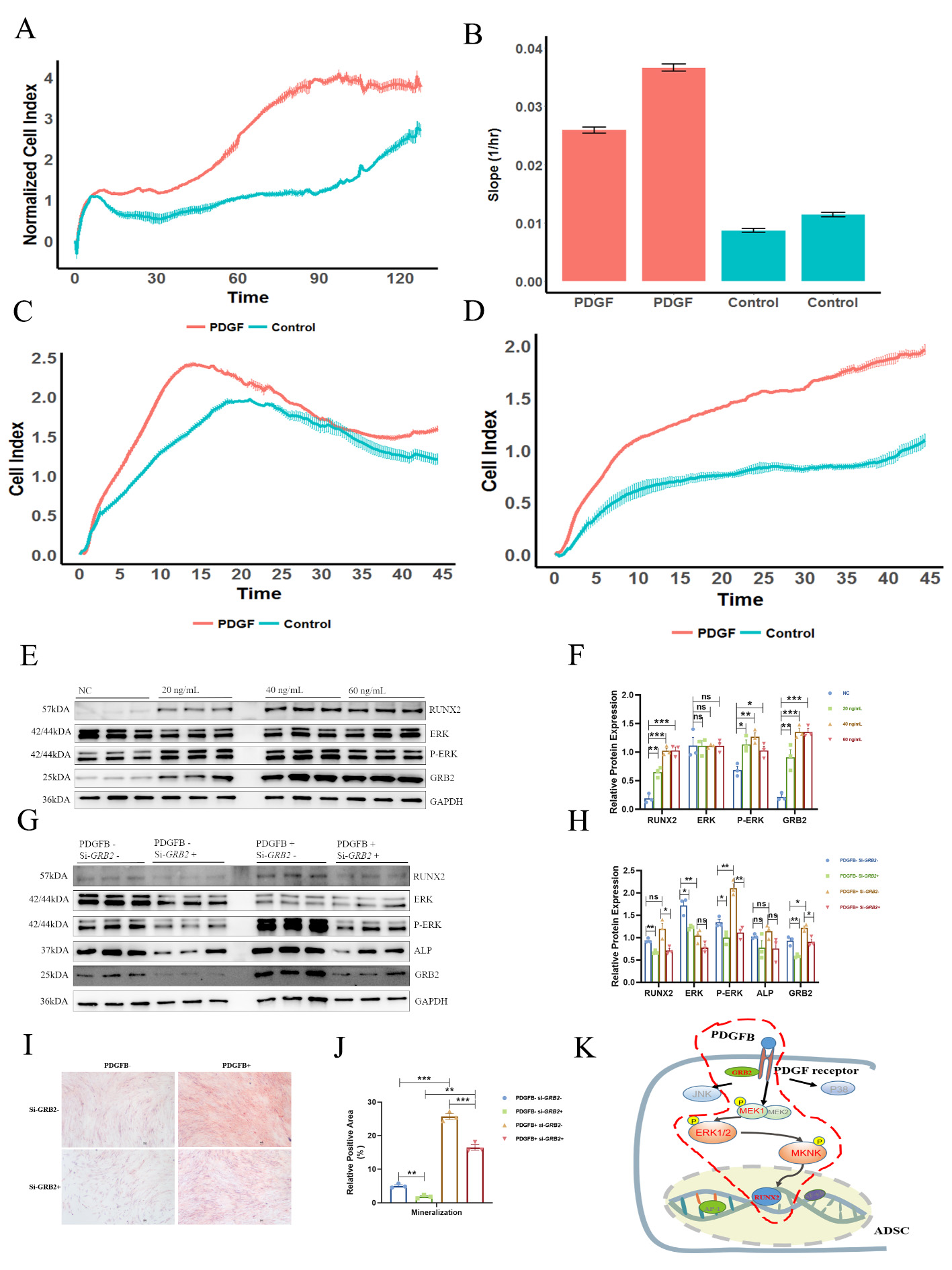
Results: The PDGF pathway was observed to have abnormal expression in the protein array, and PDGFB expression was further found to be up-regulated in 140 Chinese AS patients (Figures 1A-C). Importantly, PDGFB expression was significantly correlated with BASFI (Pearson coefficient/p value = 0.62/6.70E-8) and with the variance of the mSASSS score (mSASSS two years – baseline, Pearson coefficient/p value = 0.76/8.75E-10) (Figures 1D-E). In AS patients, preosteoclasts secreted more PDGFB than the healthy controls (p value = 1.16E-2) (Figure 1F), which could promote ADSCs osteogenesis and enhance collagen synthesis (COLI and COLIII) of osteoblasts (hFOB 1.19) (Figures 1G-H). In addition, PDGFB promoted the proliferation, chemotaxis and migration of ADSCs (Figures 2A-D). Mechanismly, in ADSCs, PDGFB stimulated ERK phosphorylation by upregulating GRB2 expression, and then increased the expression of RUNX2 to promote osteoblastogenesis of ADSCs (Figures 2E-K).
Conclusion: PDGFB stimulates the GRB2/ERK/RUNX2 pathway in ADSCs, promotes osteoblastogenesis of ADSCs, and enhances the extracellular matrix of osteoblasts, which may contribute to pathological bone formation in AS.
Y. Tang: None; Q. Liu: None; K. Tang: None; C. Geng: None; J. Xie: None; J. Wang: None; Q. Zhu: None; J. Liu: None.
Background/Purpose: Ankylosing spondylitis (AS) is a chronic inflammatory disease that mainly affects the sacroiliac joint and spine. However, the real mechanisms of immune cells acting on syndesmophyte formation in AS are not well identified. We aimed to find the key AS-associated cytokine and assess its pathogenic role in AS.
Methods: A protein array with 1000 cytokines was performed in five AS patients with the first diagnosis and five age- and gender-matched healthy controls to discover the differentially expressed cytokines. The candidate differentially expressed cytokines were further quantified by multiplex protein quantitation (3 AS-associated cytokines and 3 PDGF-pathway cytokines) and ELISA (PDGFB) in independent samples (a total of 140 AS patients vs 140 healthy controls). The effects of PDGFB, the candidate cytokine, were examined by using adipose-derived stem cells (ADSCs) and human fetal osteoblast cell line (hFOB1.19) as in vitro mesenchymal cell and preosteoblast models, respectively. Furthermore, whole-transcriptome sequencing and enrichment of phosphorylated peptides were performed by using cell models to explore the underlying mechanisms of PDGFB. The xCELLigence system was applied to examine the proliferation, chemotaxis, and migration abilities of PDGFB-stimulated or -unstimulated cells.
Results: The PDGF pathway was observed to have abnormal expression in the protein array, and PDGFB expression was further found to be up-regulated in 140 Chinese AS patients (Figures 1A-C). Importantly, PDGFB expression was significantly correlated with BASFI (Pearson coefficient/p value = 0.62/6.70E-8) and with the variance of the mSASSS score (mSASSS two years – baseline, Pearson coefficient/p value = 0.76/8.75E-10) (Figures 1D-E). In AS patients, preosteoclasts secreted more PDGFB than the healthy controls (p value = 1.16E-2) (Figure 1F), which could promote ADSCs osteogenesis and enhance collagen synthesis (COLI and COLIII) of osteoblasts (hFOB 1.19) (Figures 1G-H). In addition, PDGFB promoted the proliferation, chemotaxis and migration of ADSCs (Figures 2A-D). Mechanismly, in ADSCs, PDGFB stimulated ERK phosphorylation by upregulating GRB2 expression, and then increased the expression of RUNX2 to promote osteoblastogenesis of ADSCs (Figures 2E-K).
Conclusion: PDGFB stimulates the GRB2/ERK/RUNX2 pathway in ADSCs, promotes osteoblastogenesis of ADSCs, and enhances the extracellular matrix of osteoblasts, which may contribute to pathological bone formation in AS.

Figure 1 A, Top pathway enrichment according to the InterPro database. B, Differential expression of six cytokines in AS and healthy controls in validation I stage. C, Differential expression of PDGFB in Chinese AS patients and healthy controls in the validation II stage. AS-1 represents the 40 AS patients at baseline in the validation II stage, and AS-2 represents the 60 late-stage AS patients who have had a disease duration longer than 5 years. D, Correlation between PDGFB and BASFI. E, Correlation between PDGFB and ΔmSASSS in Chinese AS patients in validation II-40. F, Expression level of PDGFB secreted from monocytes after stimulation with M-CSF and RANKL for 7 days, which were analysed by ELISA. G, Collagen expression of FOB1.19 in the two groups (PDGF-, PDGF+). H, Relative protein level of collagen expression in FOB1.19 in the two groups (PDGF-, PDGF+). The expression levels of cytokines and the relative protein levels were compared by unpaired sample t-tests. *** P < 0.005, ** P < 0.01.

Figure 2 Effects of PDGFB on ADSCs. A, Proliferation in the two groups. B, Slope of proliferation in the two groups. C, Chemotaxis in the two groups. D, Migration results for the two groups. E-F, PDGFB activated the GRB2-pERK-RUNX2 axis in a dose-dependent manner. G-H, Western blot of several key molecules in the GRB2-pERK-RUNX2 axis with/without the treatments of PDGFB and si-GRB2. I-J, The mineralization ability of ADSCs with/without the treatments of PDGFB and si-GRB2, examined by Alizarin red S staining. K, Differential enrichment of phosphopeptides in ADSCs. MEK1, ERK1/2 and MKNK were identified as differentially phosphorylated in the MAPK pathway. The gene and protein levels were compared by unpaired sample t-tests. *** P < 0.005, ** P < 0.01, * P < 0.05.
Y. Tang: None; Q. Liu: None; K. Tang: None; C. Geng: None; J. Xie: None; J. Wang: None; Q. Zhu: None; J. Liu: None.



