Abstract Session
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Diagnosis, Manifestations, & Outcomes I: AxSpA (0841–0846)
0845: The Yield of Repeated Assessments in Chronic Back Pain Patients Suspected of Early Axial Spondyloarthritis: Two-year Data from the SPondyloArthritis Caught Early (SPACE) Cohort
Sunday, November 12, 2023
5:00 PM - 5:10 PM PT
Location: Ballroom 20A

Mary Lucy Marques, MD, MSc
Leiden University Medical Center
Coimbra, PortugalDisclosure information not submitted.
Presenting Author(s)
Mary Lucy marques1, Sofia Ramiro2, Miranda Van Lunteren3, Rosalinde Stal3, Robert BM Landewé4, Marleen van de Sande5, Karen Minde Fagerli6, Inger Jorid Berg6, Maikel van oosterhout7, Sofia Exarchou8, Roberta Ramonda9, Désirée van der Heijde2 and Floris Van Gaalen3, 1Leiden University Medical Center; Centro Hospitalar e Universitário de Coimbra (Department of Rheumatology), Coimbra, Portugal, 2Department of Rheumatology, Leiden University Medical Center, Leiden, Netherlands, 3Leiden University Medical Center, Department of Rheumatology, Leiden, Netherlands, 4Amsterdam Rheumatology & Clinical Immunology Center, Amsterdam and Zuyderland MC, Herleen, Netherlands, 5Amsterdam UMC, University of Amsterdam, Department of Rheumatology & Clinical Immunology and Department of Experimental Immunology, Amsterdam Infection & Immunity Institute; Amsterdam Rheumatology & Immunology Center (ARC), Academic Medical Center, Amsterdam, Netherlands, 6Center for Treatment of Rheumatic and Musculoskeletal Diseases (REMEDY), Diakonhjemmet Hospital, Oslo, Norway, 7Groene Hart Ziekenhuis, Gouda, Netherlands, 8Lund University, Åkarp, Sweden, 9University of Padova, Department of Rheumatology, Padova, Italy
Background/Purpose: We have shown in the SPACE cohort that a diagnosis of early axial Spondyloarthritis (axSpA) can be reliably made in patients with chronic back pain (CBP) of less than two years (2y). However, diagnostic uncertainty can be an obstacle to initiating appropriate treatment. The value of repeated assessments of SpA features for a definite diagnosis is yet to be determined. Here we aimed to assess the yield of repeated assessments of SpA features over 2y to make a definite axSpA diagnosis in patients with recent onset CBP referred to the rheumatologist, and to describe the characteristics of patients who change to definiteaxSpA over time.
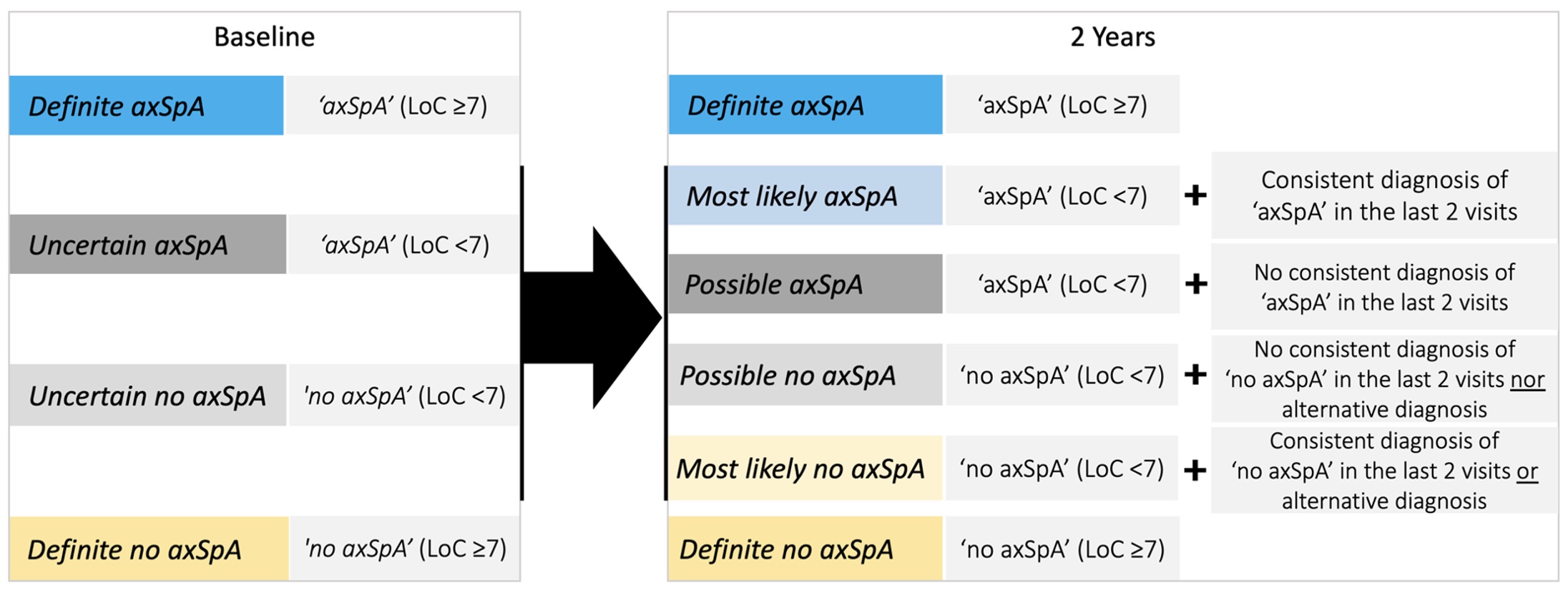
Methods: We used the 2y data from the SPACE cohort, a European multicentre inception cohort of patients (< 45y) with CBP of recent onset (≥3 months, ≤2y) included from 2008 to 2016. The diagnostic work-up consisted of patient history, physical exam, acute phase reactants (APR) and HLA-B27 testing, radiographs, and MRI of the sacroiliac joints (SI-CR and SI-MRI) and of the spine (not shown). In patients with ≥1 major or ≥2 minor prespecified SpA features, clinical assessments, APR, and imaging were repeated at 3 months, 1y and 2y visits. At each visit, the rheumatologist reported a clinical diagnosis of axSpA or no axSpA with level of confidence (LoC; numeric rating scale from 0 (not confident at all) to 10 (very confident)). Herein, we categorized patients by diagnosis likelihood (baseline and 2y definitions in Figure 1). The ASAS classification criteria were applied using local reading. We explored the diagnostic course over 2y. In patients with a new diagnosis of definite axSpA at 2y, SpA features were investigated over time.
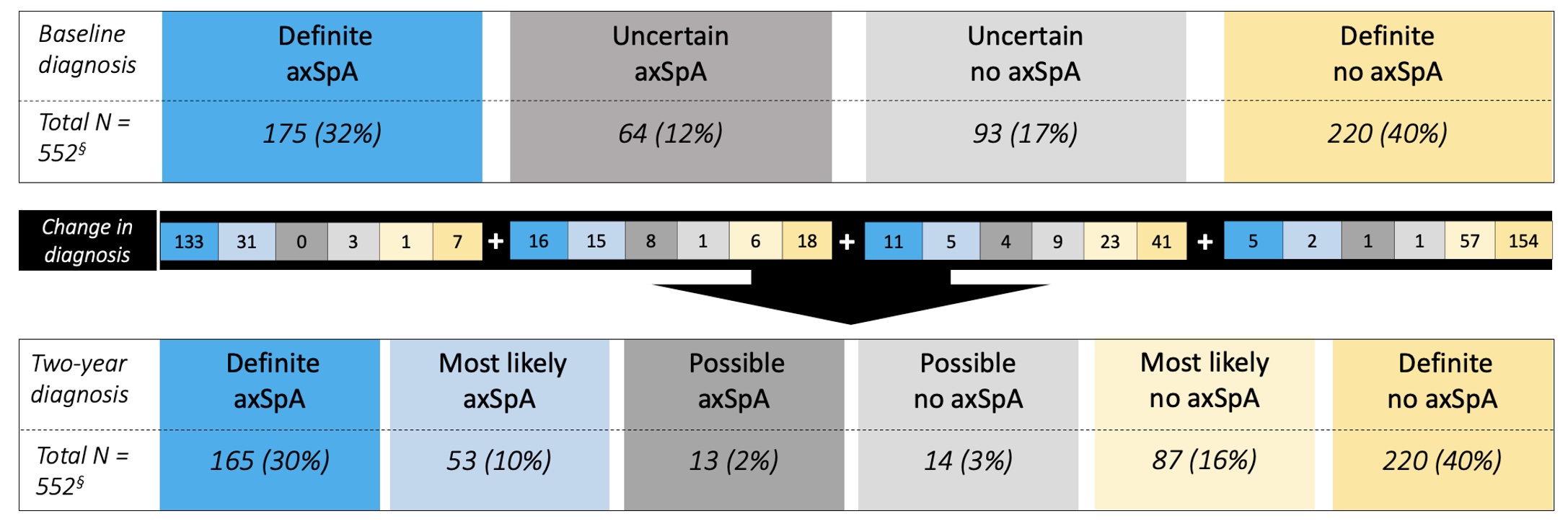
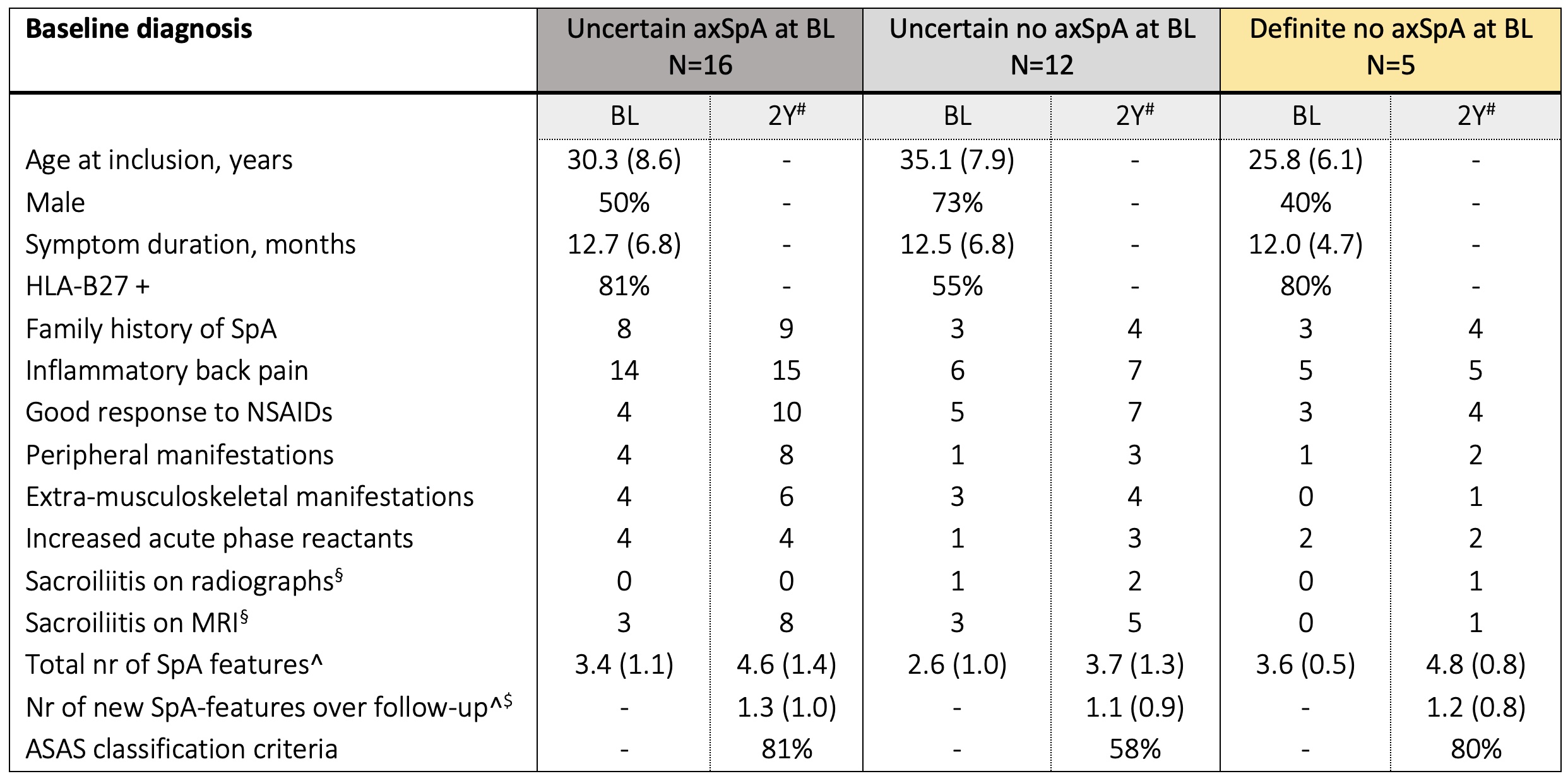
Results: We included 552 patients. Definite axSpA was attributed to 175 (32%) patients at BL and 165 (30%) at 2y (Figure 2), 155/175 (89%) and 145/165 (87%) fulfilled ASAS classification criteria, respectively. Of the 175 patients with definite axSpA at BL, 133 (76%) retained the diagnosis, and only 11 (6%) changed to no axSpA at 2y. Although still considered as axSpA by the rheumatologist, 31/175 (17%) definite axSpA patients at baseline were no longer definite axSpA at 2y, due to a decrease in LoC < 7 (n=14/31) or incomplete follow-up (n=17/31). Overall, the diagnosis changed to definite axSpA over 2y in 32 patients (BL: 16 uncertain axSpA, 11 uncertain no axSpA, and 5 definite no axSpA); on average, 3 to 4 SpA features were already present at BL and 1 new SpA feature developed over 2y (Table 1), with response to NSAIDs (9/24 patients) and MRI sacroiliitis (8/24 patients) being the most frequently developed over time. Of the 8 patients with new MRI sacroiliitis over time, 7 (88%) were HLA-B27+ and 5 (63%) were male.
Conclusion: The yield of repeated assessments of SpA features in patients with CBP suspected of axSpA was modest for the increase of new definite axSpA diagnosis at 2y. Most SpA features were already present at BL, with sacroiliitis on MRI and response to NSAIDs being the two most frequently incident SpA features potentially adding to a definite axSpA diagnosis over time. Usefulness of repeating MRI in terms of diagnostic yield is low but can be considered in HLA-B27+ patients, especially if male.
M. marques: None; S. Ramiro: AbbVie, 2, 5, Eli Lilly, 2, Galapagos, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Sanofi, 2, UCB Pharma, 2, 5; M. Van Lunteren: None; R. Stal: None; R. Landewé: AbbVie, 2, 5, AstraZeneca, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, 5, Pfizer, 2, 5, Rheumatology Consultancy BV, 12, Owner, UCB Pharma, 2, 5; M. van de Sande: AbbVie, 2, Eli Lilly, 5, Janssen, 6, Novartis, 2, 5, 6, UCB Pharma, 2, 5, 6; K. Minde Fagerli: None; I. Jorid Berg: None; M. van oosterhout: None; S. Exarchou: Amgen, 2, Janssen, 2, Novartis, 2, UCB Pharma, 2; R. Ramonda: None; D. van der Heijde: AbbVie, 2, Bayer, 2, BMS, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Imaging Rheumatology BV, 12, Director, Janssen, 2, Novartis, 2, Pfizer, 2, Takeda, 2, UCB Pharma, 2; F. Van Gaalen: AbbVie, 12, Personal fees, BMS, 12, Personal fees, Eli Lilly, 12, Personal fees, Jacobus Stichting, 5, MSD, 12, Personal fees, Novartis, 5, 12, Fees, Stichting ASAS, 5, Stichting Vrienden van Sole Mio, 5, UCB Pharma, 5.
Background/Purpose: We have shown in the SPACE cohort that a diagnosis of early axial Spondyloarthritis (axSpA) can be reliably made in patients with chronic back pain (CBP) of less than two years (2y). However, diagnostic uncertainty can be an obstacle to initiating appropriate treatment. The value of repeated assessments of SpA features for a definite diagnosis is yet to be determined. Here we aimed to assess the yield of repeated assessments of SpA features over 2y to make a definite axSpA diagnosis in patients with recent onset CBP referred to the rheumatologist, and to describe the characteristics of patients who change to definiteaxSpA over time.
Methods: We used the 2y data from the SPACE cohort, a European multicentre inception cohort of patients (< 45y) with CBP of recent onset (≥3 months, ≤2y) included from 2008 to 2016. The diagnostic work-up consisted of patient history, physical exam, acute phase reactants (APR) and HLA-B27 testing, radiographs, and MRI of the sacroiliac joints (SI-CR and SI-MRI) and of the spine (not shown). In patients with ≥1 major or ≥2 minor prespecified SpA features, clinical assessments, APR, and imaging were repeated at 3 months, 1y and 2y visits. At each visit, the rheumatologist reported a clinical diagnosis of axSpA or no axSpA with level of confidence (LoC; numeric rating scale from 0 (not confident at all) to 10 (very confident)). Herein, we categorized patients by diagnosis likelihood (baseline and 2y definitions in Figure 1). The ASAS classification criteria were applied using local reading. We explored the diagnostic course over 2y. In patients with a new diagnosis of definite axSpA at 2y, SpA features were investigated over time.
Results: We included 552 patients. Definite axSpA was attributed to 175 (32%) patients at BL and 165 (30%) at 2y (Figure 2), 155/175 (89%) and 145/165 (87%) fulfilled ASAS classification criteria, respectively. Of the 175 patients with definite axSpA at BL, 133 (76%) retained the diagnosis, and only 11 (6%) changed to no axSpA at 2y. Although still considered as axSpA by the rheumatologist, 31/175 (17%) definite axSpA patients at baseline were no longer definite axSpA at 2y, due to a decrease in LoC < 7 (n=14/31) or incomplete follow-up (n=17/31). Overall, the diagnosis changed to definite axSpA over 2y in 32 patients (BL: 16 uncertain axSpA, 11 uncertain no axSpA, and 5 definite no axSpA); on average, 3 to 4 SpA features were already present at BL and 1 new SpA feature developed over 2y (Table 1), with response to NSAIDs (9/24 patients) and MRI sacroiliitis (8/24 patients) being the most frequently developed over time. Of the 8 patients with new MRI sacroiliitis over time, 7 (88%) were HLA-B27+ and 5 (63%) were male.
Conclusion: The yield of repeated assessments of SpA features in patients with CBP suspected of axSpA was modest for the increase of new definite axSpA diagnosis at 2y. Most SpA features were already present at BL, with sacroiliitis on MRI and response to NSAIDs being the two most frequently incident SpA features potentially adding to a definite axSpA diagnosis over time. Usefulness of repeating MRI in terms of diagnostic yield is low but can be considered in HLA-B27+ patients, especially if male.

Figure 1. Definitions for diagnostic categories at baseline and at two years. If two-year visit data was missing, a last observation carried forward approach was used. axSpA – axial Spondyloarthritis; LoC – level of confidence.

Figure 2. Course of diagnosis from baseline to two years in patients with recent onset chronic back pain of unknown origin. axSpA – axial Spondyloarthritis.

Table 1. Characteristics of 32 patients changing to definite axSpA with newly developed SpA features over 2 years
Data presented as mean (SD), % or n of patients. §Local readings. ^including HLA-B27 positivity and sacroiliitis on imaging. $Of the 32 patients, 24 (75%) developed new SpA features over time. #Cumulative numbers over the two-year follow-up. BL – baseline; MRI – magnetic resonance imaging.
Data presented as mean (SD), % or n of patients. §Local readings. ^including HLA-B27 positivity and sacroiliitis on imaging. $Of the 32 patients, 24 (75%) developed new SpA features over time. #Cumulative numbers over the two-year follow-up. BL – baseline; MRI – magnetic resonance imaging.
M. marques: None; S. Ramiro: AbbVie, 2, 5, Eli Lilly, 2, Galapagos, 5, MSD, 2, 5, Novartis, 2, 5, Pfizer, 2, 5, Sanofi, 2, UCB Pharma, 2, 5; M. Van Lunteren: None; R. Stal: None; R. Landewé: AbbVie, 2, 5, AstraZeneca, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, 5, Pfizer, 2, 5, Rheumatology Consultancy BV, 12, Owner, UCB Pharma, 2, 5; M. van de Sande: AbbVie, 2, Eli Lilly, 5, Janssen, 6, Novartis, 2, 5, 6, UCB Pharma, 2, 5, 6; K. Minde Fagerli: None; I. Jorid Berg: None; M. van oosterhout: None; S. Exarchou: Amgen, 2, Janssen, 2, Novartis, 2, UCB Pharma, 2; R. Ramonda: None; D. van der Heijde: AbbVie, 2, Bayer, 2, BMS, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Imaging Rheumatology BV, 12, Director, Janssen, 2, Novartis, 2, Pfizer, 2, Takeda, 2, UCB Pharma, 2; F. Van Gaalen: AbbVie, 12, Personal fees, BMS, 12, Personal fees, Eli Lilly, 12, Personal fees, Jacobus Stichting, 5, MSD, 12, Personal fees, Novartis, 5, 12, Fees, Stichting ASAS, 5, Stichting Vrienden van Sole Mio, 5, UCB Pharma, 5.



