Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes I: Biomarkers (0847–0852)
0850: Persistence of Urinary Biomarkers of Intrarenal Inflammation Precedes Loss of Kidney Function in Lupus Nephritis
Sunday, November 12, 2023
4:45 PM - 4:55 PM PT
Location: Exhibit Hall A-B
- AF
Andrea Fava, MD
Johns Hopkins University
Baltimore, MD, United StatesDisclosure(s): Annexon Biosciences: Consultant (Ongoing); Sanofi: Advisor or Review Panel Member (Terminated, April 30, 2022)
Presenting Author(s)
Andrea Fava1, Mohamed G. Atta1, Jose Monroy-Trujillo2, Derek Fine2, Daniel Goldman3, Izmirly peter4, H Michael Belmont5, the Accelerating Medicines Partnership in RA/SLE6, Jill Buyon7 and Michelle Petri3, 1Johns Hopkins University, Baltimore, MD, 2Johns Hopkins School of Medicine, Baltimore, MD, 3Department of Medicine, Division of Rheumatology, Johns Hopkins University School of Medicine, Timonium, MD, 4NYU, New York, NY, 5NYU School of Medicine, New York, NY, 6Multiple, Multiple, 7NYU Grossman School of Medicine, New York, NY
Background/Purpose: One third of lupus nephritis (LN) patients develop irreversible kidney damage despite achieving a clinical response based on resolution of proteinuria. Furthermore, per protocol kidney biopsies in patients with proteinuria < 0.5 g/g showed clinically significant histological activity in 50% despite the minimal proteinuria. We hypothesized that persistence of intrarenal inflammation (=LN histological activity) after treatment leads to accrual of kidney damage. We have previously identified several urinary biomarkers that correlate with the NIH Activity Index (histological activity). Here, we tested whether the elevation of these candidate biomarkers of LN immunological activity at 6 and 12 months from the diagnostic kidney biopsy predict loss of kidney function at 3 years.
Methods: We quantified 1200 biomarkers (Kiloplex, RayBiotech) in urine samples collected on the day of (73%) or within 3 weeks (27%) of kidney biopsy and week 12, 24, or 52 in LN patients (ISN class III, IV, V, or mixed) with proteinuria > 1 g/d. Glomerular filtration rate (GFR) was estimated using the CKD-EPI equation. Significant GFR loss was defined as a decline of >15 ml/min below 90 ml/min at 3 years from biopsy or end-stage kidney disease (ESKD) by year 3 requiring dialysis or transplant.
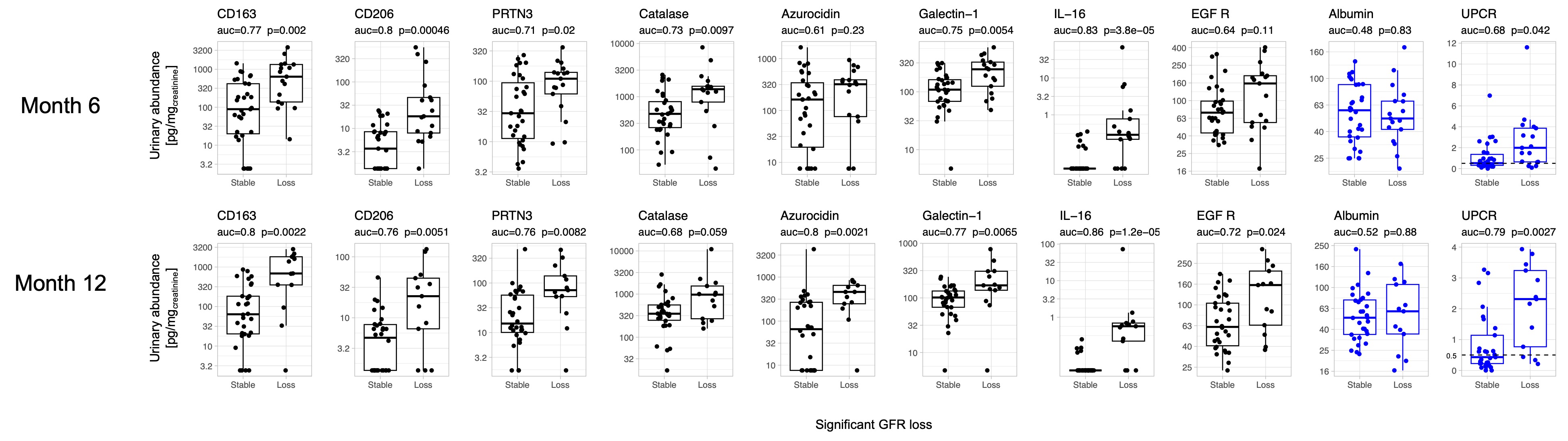
Results: We included 73 patients: 85% female, 46% identified as Black, 40% as White, 10% as Asian, and 4% as Other. ISN classification included 25% pure proliferative (III or IV), 40% pure membranous (V), and 35% mixed (III or IV + V). Mean GFR at biopsy was 85 (SD 34.7) ml/min. There were 32/73 (44%) patients who developed significant GFR loss. Figure 1 shows the associations of candidate urinary biomarkers at 6 and 12 months with significant GFR loss at 3 years. Most urinary biomarkers of histological activity were higher at 6 and 12 months in patients who ultimately lost GFR at 3 years. For example, IL-16 outperformed UPCR both at 6 and 12 months (Figure 1) and was independent of proteinuria (not shown). UPCR at 12 months predicted 3-year GFR loss with AUC 0.79, but albuminuria did not. In a multivariable model, the combination of CD163 (macrophage activation), PRTN3 (degranulation), and IL-16 (cellular inflammation in LN) urinary levels at 12 months predicted GFR loss at 3 years with an AUC of 0.96.
Conclusion: Elevation of urinary biomarkers of histological activity after 6 or 12 months of treatment predict GFR loss at 3 years better than proteinuria, especially IL-16. These findings suggest that insufficient immunosuppression results in persistent intrarenal immunological activity in LN that increases the risk of kidney function loss. The ultimate treatment goal in LN is long term preservation of kidney function. Therefore, clinical trial endpoints should include response definitions that best associate with GFR preservation. Because noninvasive urinary biomarkers of immunological activity parallel intrarenal inflammation and predict future GFR, they could be used to 1) monitor treatment response/failure, 2) allow early treatment changes, and 3) serve as surrogate endpoints in clinical trials.
A. Fava: Annexon Biosciences, 2, Sanofi, 1; M. Atta: Bayer, 5, Dimerix, 5, horizon therapeutics, 1, Morphosys AG, 5, Novartis, 5, REATA, 5, Vertex, 5; J. Monroy-Trujillo: None; D. Fine: None; D. Goldman: None; I. peter: None; H. Belmont: Alexion, 6, Aurinia, 6; t. Accelerating Medicines Partnership in RA/SLE: None; J. Buyon: Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, Related Sciences, 1; M. Petri: Alexion, 1, Amgen, 1, AnaptysBio, 1, Annexon Bio, 1, Argenx, 1, Arhros-Focus Med/Ed, 6, AstraZeneca, 1, 5, Aurinia, 1, 5, 6, Axdev, 1, Biogen, 1, Boxer Capital, 2, Cabaletto Bio, 2, Caribou Biosciences, 2, CVS Health, 1, Eli Lilly, 1, 5, Emergent Biosolutions, 1, Exagen, 5, Exo Therapeutics, 2, Gilead Biosciences, 2, GlaxoSmithKlein(GSK), 1, 5, 6, Horizon Therapeutics, 2, Idorsia Pharmaceuticals, 2, IQVIA, 1, Janssen, 1, 5, Kira Pharmaceuticals, 2, MedShr, 6, Merck/EMD Serono, 1, Momenta Pharmaceuticals, 2, Nexstone Immunology, 2, Nimbus Lakshmi, 2, Proviant, 2, Sanofi, 2, Sinomab Biosciences, 2, Thermofisher, 5, UCB, 2.
Background/Purpose: One third of lupus nephritis (LN) patients develop irreversible kidney damage despite achieving a clinical response based on resolution of proteinuria. Furthermore, per protocol kidney biopsies in patients with proteinuria < 0.5 g/g showed clinically significant histological activity in 50% despite the minimal proteinuria. We hypothesized that persistence of intrarenal inflammation (=LN histological activity) after treatment leads to accrual of kidney damage. We have previously identified several urinary biomarkers that correlate with the NIH Activity Index (histological activity). Here, we tested whether the elevation of these candidate biomarkers of LN immunological activity at 6 and 12 months from the diagnostic kidney biopsy predict loss of kidney function at 3 years.
Methods: We quantified 1200 biomarkers (Kiloplex, RayBiotech) in urine samples collected on the day of (73%) or within 3 weeks (27%) of kidney biopsy and week 12, 24, or 52 in LN patients (ISN class III, IV, V, or mixed) with proteinuria > 1 g/d. Glomerular filtration rate (GFR) was estimated using the CKD-EPI equation. Significant GFR loss was defined as a decline of >15 ml/min below 90 ml/min at 3 years from biopsy or end-stage kidney disease (ESKD) by year 3 requiring dialysis or transplant.
Results: We included 73 patients: 85% female, 46% identified as Black, 40% as White, 10% as Asian, and 4% as Other. ISN classification included 25% pure proliferative (III or IV), 40% pure membranous (V), and 35% mixed (III or IV + V). Mean GFR at biopsy was 85 (SD 34.7) ml/min. There were 32/73 (44%) patients who developed significant GFR loss. Figure 1 shows the associations of candidate urinary biomarkers at 6 and 12 months with significant GFR loss at 3 years. Most urinary biomarkers of histological activity were higher at 6 and 12 months in patients who ultimately lost GFR at 3 years. For example, IL-16 outperformed UPCR both at 6 and 12 months (Figure 1) and was independent of proteinuria (not shown). UPCR at 12 months predicted 3-year GFR loss with AUC 0.79, but albuminuria did not. In a multivariable model, the combination of CD163 (macrophage activation), PRTN3 (degranulation), and IL-16 (cellular inflammation in LN) urinary levels at 12 months predicted GFR loss at 3 years with an AUC of 0.96.
Conclusion: Elevation of urinary biomarkers of histological activity after 6 or 12 months of treatment predict GFR loss at 3 years better than proteinuria, especially IL-16. These findings suggest that insufficient immunosuppression results in persistent intrarenal immunological activity in LN that increases the risk of kidney function loss. The ultimate treatment goal in LN is long term preservation of kidney function. Therefore, clinical trial endpoints should include response definitions that best associate with GFR preservation. Because noninvasive urinary biomarkers of immunological activity parallel intrarenal inflammation and predict future GFR, they could be used to 1) monitor treatment response/failure, 2) allow early treatment changes, and 3) serve as surrogate endpoints in clinical trials.

Figure 1. Association of candidate urinary biomarkers of histological activity with significant GFR loss. Urinary abundance (pg/mgcreatinine) of urinary biomarkers selected a priori based on their correlation with histological activity (NIH Activity Index) in a matching kidney biopsy of LN according to GFR loss at 3 years. Urine protein and albumin to creatinine ratios are reported for reference as clinically used biomarkers.
A. Fava: Annexon Biosciences, 2, Sanofi, 1; M. Atta: Bayer, 5, Dimerix, 5, horizon therapeutics, 1, Morphosys AG, 5, Novartis, 5, REATA, 5, Vertex, 5; J. Monroy-Trujillo: None; D. Fine: None; D. Goldman: None; I. peter: None; H. Belmont: Alexion, 6, Aurinia, 6; t. Accelerating Medicines Partnership in RA/SLE: None; J. Buyon: Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, Related Sciences, 1; M. Petri: Alexion, 1, Amgen, 1, AnaptysBio, 1, Annexon Bio, 1, Argenx, 1, Arhros-Focus Med/Ed, 6, AstraZeneca, 1, 5, Aurinia, 1, 5, 6, Axdev, 1, Biogen, 1, Boxer Capital, 2, Cabaletto Bio, 2, Caribou Biosciences, 2, CVS Health, 1, Eli Lilly, 1, 5, Emergent Biosolutions, 1, Exagen, 5, Exo Therapeutics, 2, Gilead Biosciences, 2, GlaxoSmithKlein(GSK), 1, 5, 6, Horizon Therapeutics, 2, Idorsia Pharmaceuticals, 2, IQVIA, 1, Janssen, 1, 5, Kira Pharmaceuticals, 2, MedShr, 6, Merck/EMD Serono, 1, Momenta Pharmaceuticals, 2, Nexstone Immunology, 2, Nimbus Lakshmi, 2, Proviant, 2, Sanofi, 2, Sinomab Biosciences, 2, Thermofisher, 5, UCB, 2.




