Abstract Session
Immunobiology
Session: Abstracts: T Cell Biology & Targets in Autoimmune & Inflammatory Disease (0799–0804)
0801: LncRNA PIGL-217 Regulates Th17 Differentiation by Targeting miR-5008-5p and Suppressing FoxO1 in Behçet's Disease
Sunday, November 12, 2023
4:30 PM - 4:40 PM PT
Location: Room 30D-E
- ZW
Zhimian Wang, MB (she/her/hers)
Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College
Beijing, Beijing, ChinaDisclosure information not submitted.
Presenting Author(s)
Zhimian Wang, Xin Yu, Hua Chen and Wenjie Zheng, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Beijing, China
Background/Purpose: Dysregulated Th17 cells are implicated in Behçet's disease (BD). However, the underlying mechanism remains unclear. Here we aim to elucidate the mechanism of forkhead box O1 (FoxO1) and its regulating long non-coding RNA (lncRNA) in modulating Th17 cells in BD.
Methods: Th17 cells in peripheral blood mononuclear cells (PBMCs) from BD and healthy controls (HCs) were analyzed by flow cytometry. Naïve CD4+T cells from both groups were isolated for further experiments. Microarray analysis was performed on naïve CD4+T cells of six BD patients and six gender- and age-matched HCs to gain insight into their respective gene expression profiles, which was validated by qRT-PCR analyses. To investigate the role of lncRNA in Th17 differentiation, naïve CD4+T cells were transfected using siRNA, overexpression plasmids or miRNA mimics as required, and were incubated under Th17-polarizing condition for 5 days. A dual-luciferase reporter assay was used to confirm the target of miRNA.
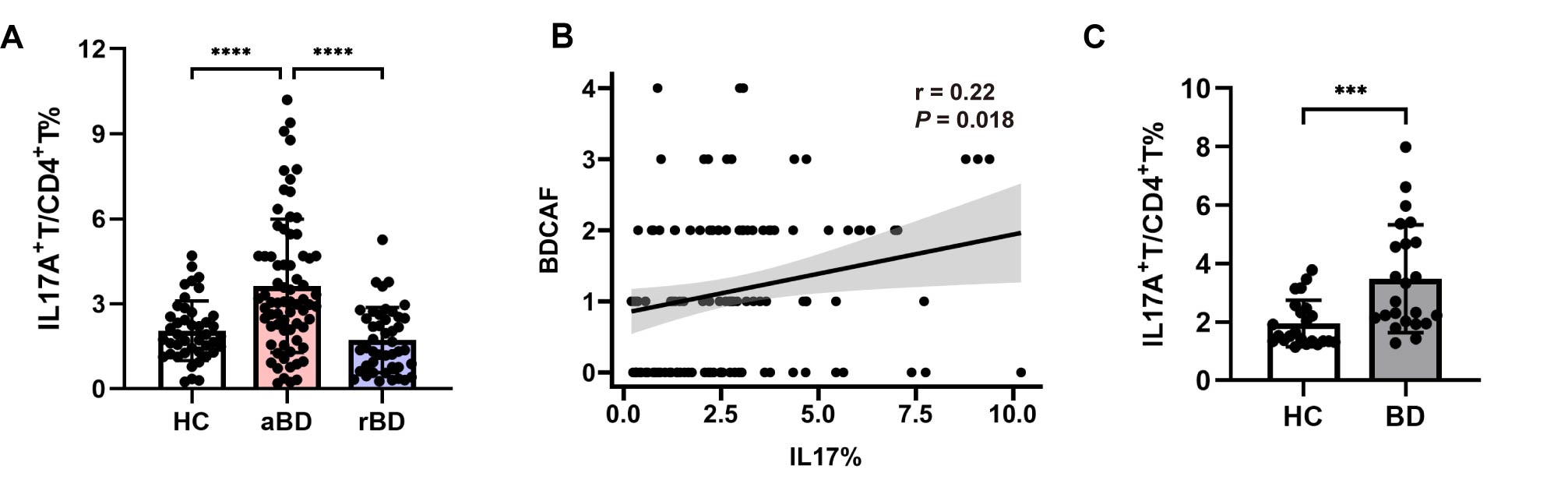
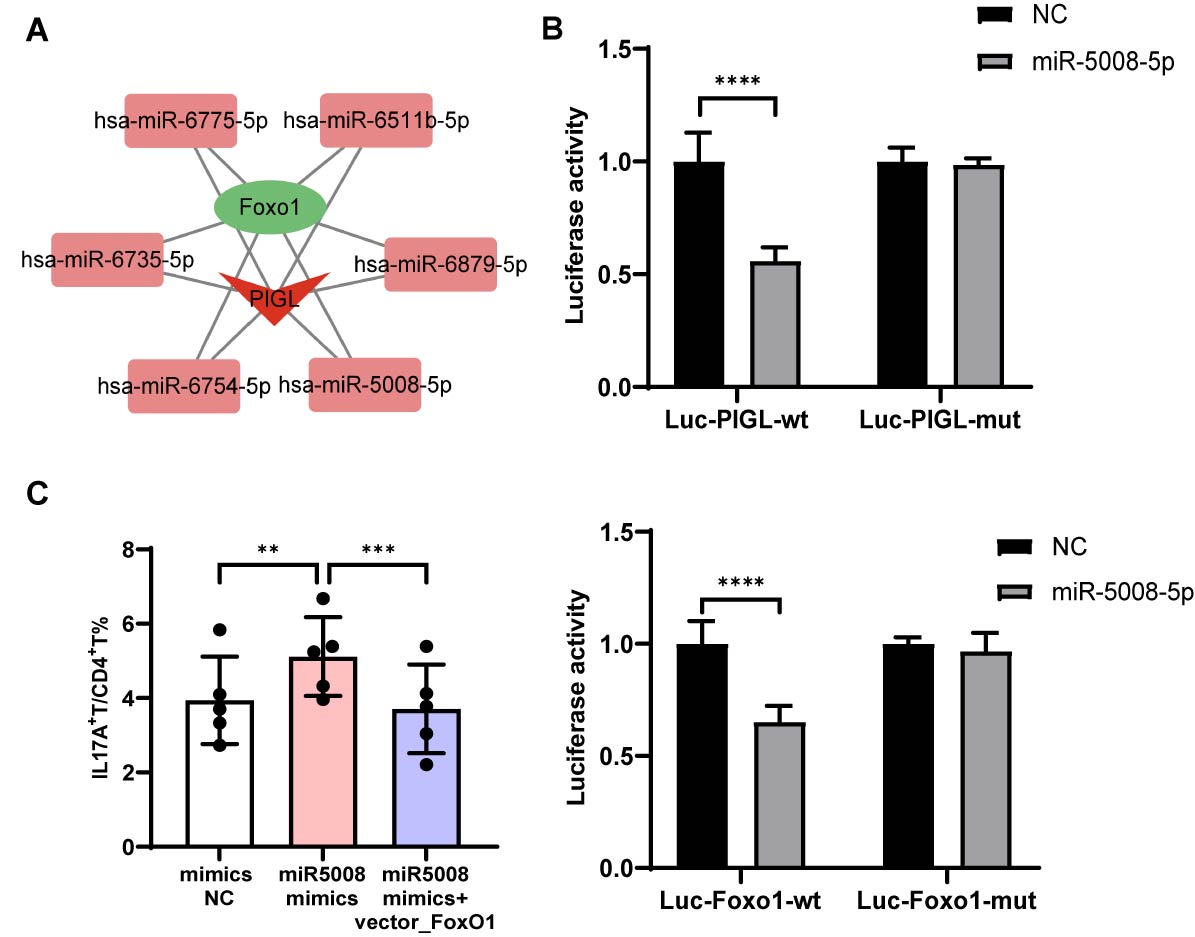
Results: Circulating Th17 cells were elevated in active BD patients (p< 0.0001), which were positively correlated with BDCAF. BD naïve CD4+T cells showed enhanced Th17 cell differentiation capacity compared to HC cells (p< 0.001) (Fig. 1). Microarray analysis revealed that Th17-related FoxO signaling pathway was inhibited in BD naïve CD4+T cells, along with downregulation of FoxO1 and its related lncRNA PIGL-217 (Fig. 2A-B), which was confirmed in vitro (p< 0.0001 and p< 0.001, respectively). We further demonstrated that PIGL-217 silencing in naïve CD4+T cells decreased FoxO1 expression (p< 0.05) and enhanced Th17 differentiation (p< 0.05), which was attenuated by FoxO1 overexpression (p< 0.01) (Fig. 2C). Using competing endogenous RNA (ceRNA) prediction, we identified miR-5008-5p as a candidate gene (Fig. 3A), with increased expression in BD naïve CD4+T cells (p< 0.0001). Dual-Luciferase reporter assay confirmed FoxO1 and PIGL-217 were targeted by miR-5008-5p (Fig. 3B), the expression of which was significantly increased after PIGL-217 knockdown (p< 0.05). Finally, miR-5008-5p mimics significantly down-regulated FoxO1 expression (p< 0.001) and promoted Th17 differentiation (p< 0.01), which was reversed by PIGL-217 overexpression (p< 0.001) (Fig. 3C).
Conclusion: Our data suggest that Th17 cell differentiation is enhanced and plays a role in BD. We identify PIGL-217 as a novel lncRNA regulating Th17 differentiation, which acts as a sponge for miR-5008-5p to regulate FoxO1 expression via the ceRNA mechanism.
.jpg)
Z. Wang: None; X. Yu: None; H. Chen: None; W. Zheng: None.
Background/Purpose: Dysregulated Th17 cells are implicated in Behçet's disease (BD). However, the underlying mechanism remains unclear. Here we aim to elucidate the mechanism of forkhead box O1 (FoxO1) and its regulating long non-coding RNA (lncRNA) in modulating Th17 cells in BD.
Methods: Th17 cells in peripheral blood mononuclear cells (PBMCs) from BD and healthy controls (HCs) were analyzed by flow cytometry. Naïve CD4+T cells from both groups were isolated for further experiments. Microarray analysis was performed on naïve CD4+T cells of six BD patients and six gender- and age-matched HCs to gain insight into their respective gene expression profiles, which was validated by qRT-PCR analyses. To investigate the role of lncRNA in Th17 differentiation, naïve CD4+T cells were transfected using siRNA, overexpression plasmids or miRNA mimics as required, and were incubated under Th17-polarizing condition for 5 days. A dual-luciferase reporter assay was used to confirm the target of miRNA.
Results: Circulating Th17 cells were elevated in active BD patients (p< 0.0001), which were positively correlated with BDCAF. BD naïve CD4+T cells showed enhanced Th17 cell differentiation capacity compared to HC cells (p< 0.001) (Fig. 1). Microarray analysis revealed that Th17-related FoxO signaling pathway was inhibited in BD naïve CD4+T cells, along with downregulation of FoxO1 and its related lncRNA PIGL-217 (Fig. 2A-B), which was confirmed in vitro (p< 0.0001 and p< 0.001, respectively). We further demonstrated that PIGL-217 silencing in naïve CD4+T cells decreased FoxO1 expression (p< 0.05) and enhanced Th17 differentiation (p< 0.05), which was attenuated by FoxO1 overexpression (p< 0.01) (Fig. 2C). Using competing endogenous RNA (ceRNA) prediction, we identified miR-5008-5p as a candidate gene (Fig. 3A), with increased expression in BD naïve CD4+T cells (p< 0.0001). Dual-Luciferase reporter assay confirmed FoxO1 and PIGL-217 were targeted by miR-5008-5p (Fig. 3B), the expression of which was significantly increased after PIGL-217 knockdown (p< 0.05). Finally, miR-5008-5p mimics significantly down-regulated FoxO1 expression (p< 0.001) and promoted Th17 differentiation (p< 0.01), which was reversed by PIGL-217 overexpression (p< 0.001) (Fig. 3C).
Conclusion: Our data suggest that Th17 cell differentiation is enhanced and plays a role in BD. We identify PIGL-217 as a novel lncRNA regulating Th17 differentiation, which acts as a sponge for miR-5008-5p to regulate FoxO1 expression via the ceRNA mechanism.

Fig. 1 Aberrant Th17 cells in BD patients. (A) Level of IL-17A+T cells in peripheral CD4+T cells of HC (n=43), active (n=71) and inactive (n=44) BD patients. (B) Pearson correlation of BDCAF with frequency of circulating IL-17A+T cells. (C) Level of IL-17A+T cells induced from HC (n=23) and BD (n=23) naïve CD4+T cells under Th17-polarizing condition for 5 days. ***p < 0.001, ****p < 0.0001.
.jpg)
Fig. 2 Dysregulated PIGL-217 and FoxO1 in BD naïve CD4+T cells. (A) GSEA pathway analysis for differentially expressed mRNAs of BD naïve CD4+T cells. (B) Co-expression network of FoxO1 with associated 25 lncRNAs. (C) Level of Th17 differentiation with PIGL knockdown and FoxO1 overexpression. *p < 0.05, **p < 0.01.

Fig. 3 PIGL-miR-5008-5p-FoxO1 ceRNA network. (A) Co-expression network of FoxO1 and PIGL-217 with associated 6 miRNAs. (B) Luciferase reporter activity of PIGL-217 (up) and FoxO1-3′UTR (down) in HEK-293T cells co-transfected with miR-5008-5p mimics or mimics NC. (C) Level of Th17 differentiation transfected with miR-5008-5p mimics alone or co-transfected with FoxO1 overexpression vector. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Z. Wang: None; X. Yu: None; H. Chen: None; W. Zheng: None.



