Abstract Session
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: Abstracts: Pediatric Rheumatology – Clinical I: JIA (0829–0834)
0831: Two- and Three-Year Outcomes from the Childhood Arthritis and Rheumatology Research Alliance Start Time Optimization of Biologic Therapy in Polyarticular JIA (STOP-JIA) Study
Sunday, November 12, 2023
4:30 PM - 4:40 PM PT
Location: Room 7A-B
- SR
Sarah Ringold, MD, MS
Seattle Children's; Janssen R&D
Seattle, WA, United StatesDisclosure information not submitted.
Presenting Author(s)
Sarah Ringold1, George Tomlinson2, Laura Schanberg3, vincent del gaizo4, Katherine Murphy5, Brian Feldman6, Mei-Sing Ong7, Marc Natter8, Yukiko Kimura9 and For The CARRA Registry Investgators4, 1Seattle Children's Hospital, Seattle, WA, 2University of Toronto, Toronto, ON, Canada, 3Duke University School of Medicine, Durham, NC, 4CARRA, Inc, Washington, DC, 5Non-clinical, New Orleans, LA, 6The Hospital for Sick Children, Toronto, ON, Canada, 7Harvard Medical School & Harvard Pilgrim Healthcare Institute, Boston, MA, 8Harvard Medical School, Boston, MA, 9Hackensack Meridian School of Medicine, New York, NY
Background/Purpose: The STOP-JIA study was designed to compare the effectiveness of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Consensus Treatment Plans (CTPs) for untreated polyarticular JIA (pJIA) in achieving ACR clinically inactive disease (CID) at 1 year. The CTPs differ in the timing of initiation of biologic disease modifying anti-rheumatic drug therapy (bDMARD). The objective of this study was to measure the impact of CARRA STOP-JIA CTPs on clinical outcomes at 2 and 3 years.
Methods: STOP-JIA compared 3 CARRA CTPs in 400 children with pJIA: 1) Step-Up (SU) – starting conventional, synthetic DMARD monotherapy (csDMARD), adding bDMARD if needed after 3 months; 2) Early Combination (EC) – starting csDMARD and bDMARD within the first 3 months; and 3)Biologic First (BF) – starting bDMARD monotherapy and adding csDMARD if needed after 3 months. There was no randomization. Patients with 2 to 3 years of follow-up were included. The primary outcome was the percentage of children achieving CID off glucocorticoids at 2 and/or 3 years. Propensity score (PS) weighting was used to balance baseline differences in potential confounders between CTPs. Secondary outcomes included comparison of proportions of patients with clinical Juvenile Arthritis Disease Activity Score based on 10 joints inactive disease (cJADAS10-ID £ 2.5), clinical remission on medications (CRM; consecutive visits with CID ³ 6 months), and proportion of time spent in CID or cJADAS10-ID.
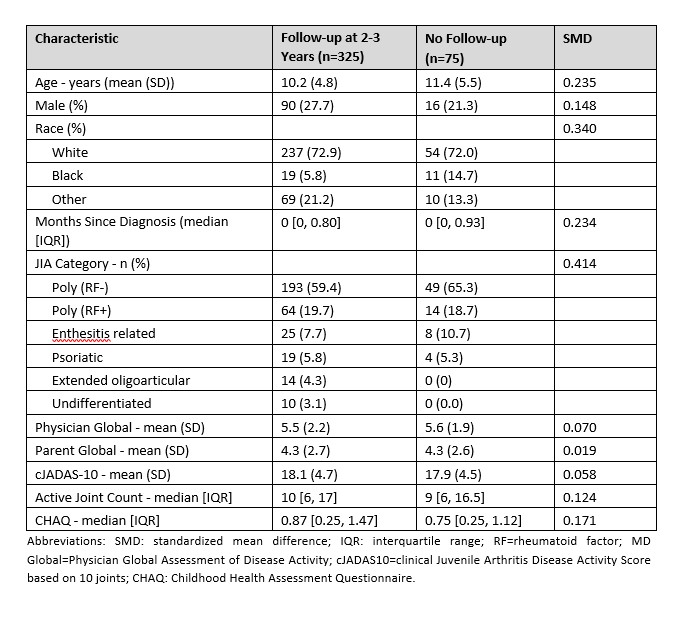
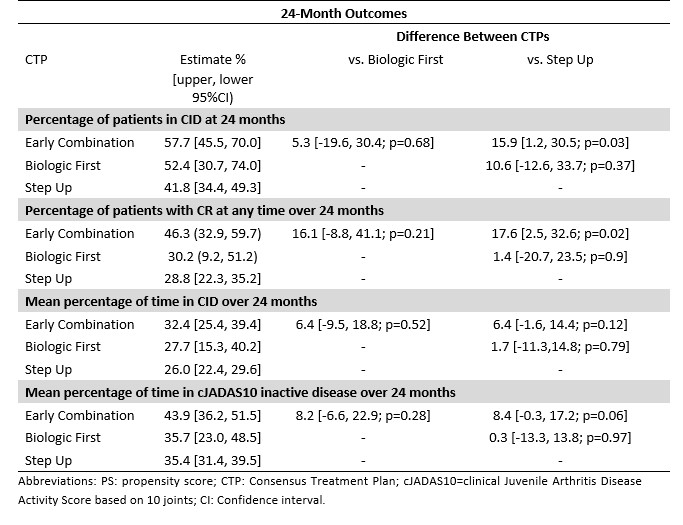
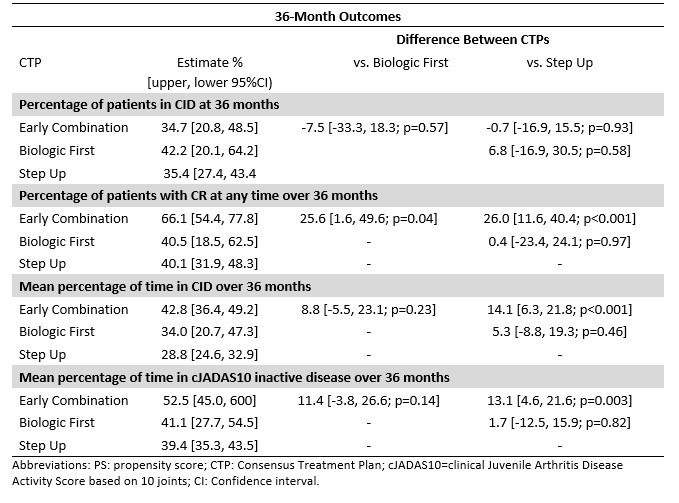
Results: 325 participants had a 2- and/or 3-year visit (n=210 SU, 83 EC, 32 BF; Table 1). Percentage of patients in CID at 2 years was 42% for SU, 58% EC, and 52% BF (p=0.03 for SU versus EC; Table 2). CID differences were not statistically significant at 3 years. Likewise, there was no significant difference between CTPs for JADAS10-ID at 2 or 3 years. However, there were significant percentage differences in CRM, which were higher for EC compared to SU over 2 years (46.3% versus 28.8% [p=0.02]) and 3 years (66.1% versus 40.1% [p< 0.01]), and for percentages of time spent in CID (42.8% versus 28.8% [p< 0.01]) and cJADAS10-ID (52.5% versus 39.4% [p< 0.01]) at 3 years.
Conclusion: These data support improved effectiveness of EC versus SU and BF at 2 and 3 years for most outcomes, but did not reach statistical significance for all comparisons. There were significant differences favoring EC versus SU in outcomes that reflected duration of time spent with less disease activity at 2 and 3 years, which may be most important in limiting disease burden. More research is needed to improve understanding of which pJIA patients will respond to a particular CTP in order to optimize individual outcomes.
S. Ringold: Janssen Research & Development, LLC, 3; G. Tomlinson: Editas Medicine Inc, 1, Spectral Medical Inc, 7; L. Schanberg: Bristol-Myers Squibb(BMS), 5, Sanofi, 12, DSMB, UCB, 12, DSMB; v. del gaizo: None; K. Murphy: None; B. Feldman: AB2Bio, 2, Janssen, 2, Novo Nordisk, 2, Pfizer, 2; M. Ong: Pfizer, 5; M. Natter: None; Y. Kimura: None; F. The CARRA Registry Investgators: None.
Background/Purpose: The STOP-JIA study was designed to compare the effectiveness of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Consensus Treatment Plans (CTPs) for untreated polyarticular JIA (pJIA) in achieving ACR clinically inactive disease (CID) at 1 year. The CTPs differ in the timing of initiation of biologic disease modifying anti-rheumatic drug therapy (bDMARD). The objective of this study was to measure the impact of CARRA STOP-JIA CTPs on clinical outcomes at 2 and 3 years.
Methods: STOP-JIA compared 3 CARRA CTPs in 400 children with pJIA: 1) Step-Up (SU) – starting conventional, synthetic DMARD monotherapy (csDMARD), adding bDMARD if needed after 3 months; 2) Early Combination (EC) – starting csDMARD and bDMARD within the first 3 months; and 3)Biologic First (BF) – starting bDMARD monotherapy and adding csDMARD if needed after 3 months. There was no randomization. Patients with 2 to 3 years of follow-up were included. The primary outcome was the percentage of children achieving CID off glucocorticoids at 2 and/or 3 years. Propensity score (PS) weighting was used to balance baseline differences in potential confounders between CTPs. Secondary outcomes included comparison of proportions of patients with clinical Juvenile Arthritis Disease Activity Score based on 10 joints inactive disease (cJADAS10-ID £ 2.5), clinical remission on medications (CRM; consecutive visits with CID ³ 6 months), and proportion of time spent in CID or cJADAS10-ID.
Results: 325 participants had a 2- and/or 3-year visit (n=210 SU, 83 EC, 32 BF; Table 1). Percentage of patients in CID at 2 years was 42% for SU, 58% EC, and 52% BF (p=0.03 for SU versus EC; Table 2). CID differences were not statistically significant at 3 years. Likewise, there was no significant difference between CTPs for JADAS10-ID at 2 or 3 years. However, there were significant percentage differences in CRM, which were higher for EC compared to SU over 2 years (46.3% versus 28.8% [p=0.02]) and 3 years (66.1% versus 40.1% [p< 0.01]), and for percentages of time spent in CID (42.8% versus 28.8% [p< 0.01]) and cJADAS10-ID (52.5% versus 39.4% [p< 0.01]) at 3 years.
Conclusion: These data support improved effectiveness of EC versus SU and BF at 2 and 3 years for most outcomes, but did not reach statistical significance for all comparisons. There were significant differences favoring EC versus SU in outcomes that reflected duration of time spent with less disease activity at 2 and 3 years, which may be most important in limiting disease burden. More research is needed to improve understanding of which pJIA patients will respond to a particular CTP in order to optimize individual outcomes.

Table 1: Patient characteristics at enrollment for participants with 2- and/or 3- year data

Table 2: Propensity Score-adjusted difference between CTP groups in estimates of CID at 24 months, CRM at any time up to 24 months, and percentages of time spent in CID and JADAS10-ID over 24 months

Table 3: Propensity Score-adjusted difference between CTP groups in estimates of CID at 36 months, CRM at any time up to 36 months, and percentages of time spent in CID and JADAS10-ID over 36 months
S. Ringold: Janssen Research & Development, LLC, 3; G. Tomlinson: Editas Medicine Inc, 1, Spectral Medical Inc, 7; L. Schanberg: Bristol-Myers Squibb(BMS), 5, Sanofi, 12, DSMB, UCB, 12, DSMB; v. del gaizo: None; K. Murphy: None; B. Feldman: AB2Bio, 2, Janssen, 2, Novo Nordisk, 2, Pfizer, 2; M. Ong: Pfizer, 5; M. Natter: None; Y. Kimura: None; F. The CARRA Registry Investgators: None.



