Abstract Session
Vasculitis
Session: Abstracts: Vasculitis – ANCA-Associated I (0853–0858)
0854: Long-term Efficacy of Remission-induction Regimens for Eosinophilic Granulomatosis with Polyangiitis
Sunday, November 12, 2023
4:15 PM - 4:25 PM PT
Location: Ballroom 20D
- MD
Presenting Author(s)
Martin Dutertre1, Gregory Pugnet2, Claire De Moreuil3, Bernard Bonnotte4, YGAL BENHAMOU5, Dominique Chauveau6, Elisabeth Diot7, Pierre Duffau8, Nicolas Limal9, Antoine Néel10, GEOFFREY URBANSKI11, Noémie Jourde-Chiche12, Nicolas MARTIN SILVA13, Francois Maurier14, Arsène Mekinian15, Nicolas Schleinitz16, Felix ackermann17, Anne-Laure Fauchais18, Antoine Froissart19, Thomas Le Gallou20, Yurdagul Uzunhan21, Jean-Francois Viallard22, Alice Berezne23, laurent chiche24, Bruno Crestani25, Guillaume Direz26, Cecile-Audrey DUREL27, Pascal Godmer28, Jean-Emmanuel Kahn29, Marc Lambert30, Mathilde de Menthon1, Thomas Quemeneur31, Jacques Cadranel1, Pierre Charles32, Antoine Dossier1, Loic Guillevin33, Xavier Puéchal34 and Benjamin Terrier35, 1AP-HP, Paris, France, 2CHU Toulouse Rangueil Service de Medecine Interne et Immunologie Clinique, Toulouse, France, 3CHU de Brest, Brest, France, 4Department of Internal Medicine and Clinical Immunology, Dijon University Hospital, Dijon, France, 5rouen university hospital, Rouen, France, 6Hôpital Le Tripode, Bordeaux, France, 7Service de médecine interne et immunologie clinique, CHU Tours, Tours, France, 8CHU Bordeaux, Bordeaux, France, 9AP-HP, Créteil, France, 10CHU de Nantes, Nantes, France, 11CHU Angers, Angers, France, 12AP-HM, Marseille, France, 13CHU Caen, Caen, France, 14Hôpitaux privés de Metz, Vaux / Frankreich, France, 15Department of Internal Medicine, Hôpital Saint-Antoine, AP-HP, Paris, France, 16Aix Marseille university, AP-HM, Marseille, France, 17Hôpital Foch, Suresnes, France, 18Dupuytren Hospital, Limoges, France, 19CHI Créteil, Créteil, France, 20CHU Rennes, Rennes, France, 21AP-HP, Bobigny, France, 22CHU de Bordeaux, Hôpital Haut-Lévêque, Pessac, France, 23CH Annecy, Annecy, France, 24Hopital Europeen, Marseille, France, 25Hopital Bichat, Paris University, Paris, France, 26CH Le Mans, Le Mans, France, 27Hospices Civils de Lyon, Lyon, France, 28CH Bretagne Atlantique, Vannes, France, 29AP-HP, Suresnes Cedex, France, 30CHRU Lille, Lille, France, 31CH Valenciennes, Valenciennes, France, 32Institut Mutualiste Montsouris, Service de Médecine Interne, Paris, France, 33University Paris Descartes, Paris, France, 34National Referral Center for Rare Systemic Autoimmune Diseases, Paris, France, 35Department of Internal Medicine, Hôpital Cochin, AP-HP, Paris, France
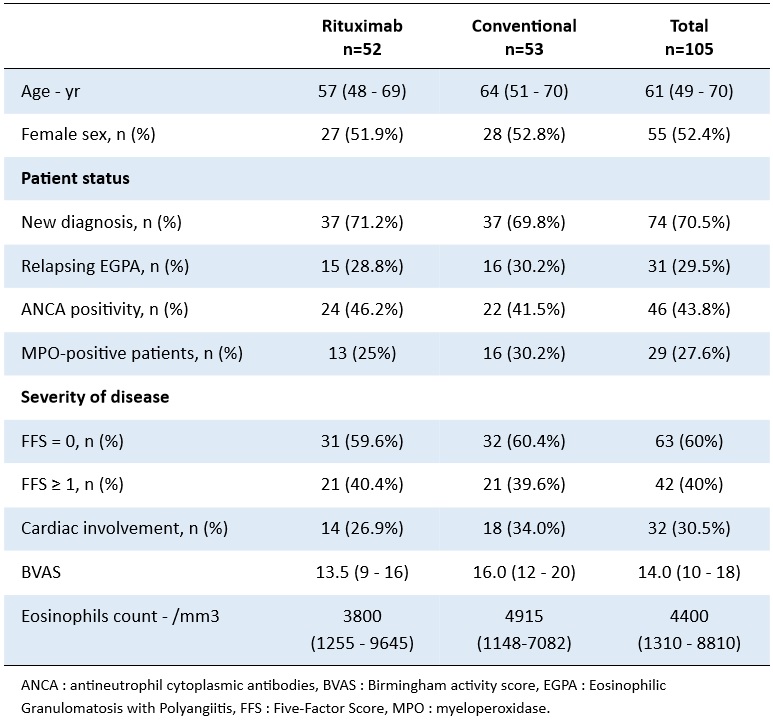
Background/Purpose: The Rituximab in Eosinophilic Granulomatosis With Polyangiitis (REOVAS) trial compared rituximab (RTX) infusions to conventional strategy for remission-induction in eosinophilic granulomatosis with polyangiitis (EGPA). This trial failed to show a superiority of RTX over the conventional strategy to induce remission at 180 days. However, the long-term efficacy and safety of RTX as compared with conventional strategy in EGPA remains unknown. We report here the long-term results of the REOVAS trial.
Methods: After completion of the 12-month REOVAS trial, patients were followed prospectively, with data on disease activity, medications and adverse events collected by the patients' physicians every 6 months until the last follow-up. The primary endpoint was the minor and major relapse-free survival. Secondary endpoints were major relapses and asthma and ENT exacerbations were also assessed.
Results: Among 105 enrolled patients, only one was lost to follow-up. The median follow-up was 45 months (IQR 34-53).
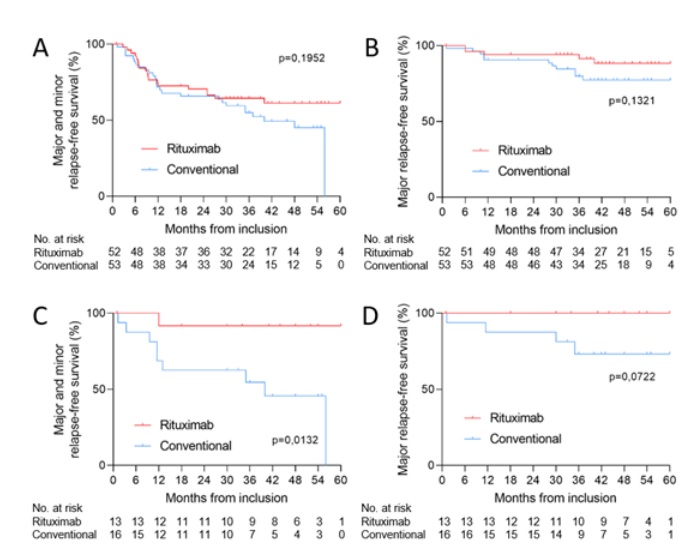
At month 45 (median follow-up), for the RTX and conventional strategy arms, respectively, the minor and major relapse-free survival rates were 63.5% (95%CI 49.9%-75.2%) and 50.9% (95%CI 37.9%-63.9%) (p=0.24) ; major relapse-free survival rates were 90.4% (95%CI 79.4%-95.8%) and 79.2% (95%CI 66.5%-88.0%) (p=0.17); asthma and/or ENT exacerbation-free survival rates were 42.3% (95%CI 29.9%-55.8%) and 34.0% (95%CI 22.7%-47.4%) (p=0.42) ; and overall EGPA-related event-free survival rates were 32.7% (95%CI 21.5%-46.2%) and 22.6% (95%CI 13.5%-35.5%) (p=0.28). We then focused our analysis on patients with MPO-ANCA and showed that the minor and major relapse-free survival rates were 92.3% (95% CI 66.7% - 99.6%) and 50% (95% CI 58% - 72%) for the RTX and conventional arms, respectively (p=0.02).
As 39 patients were enrolled during follow-up in the double-blind MAINRITSEG trial evaluating RTX versus azathioprine for maintenance in EGPA, we focused our analysis on the 66 patients (63%) who were not enrolled in MAINRITSEG. At month 45, the minor and major relapse-free survival rates for the RTX (n=30) and conventional strategy (n=36) arms were 60.0% (95%CI 42.3%-75.4%) and 38.9% (95%CI 24.8%-55.2%), respectively (p=0.14), and the major relapse-free survival rates were 90.0% (95%CI 73.6%-97.3%) and 72.2% (95%CI 55.9%-84.3%), respectively (p=0.12). When analyzing only ANCA-positive patients (14 in the RTX and 14 in the conventional arms), the minor and major relapse-free survival rates were 78.6% (95%CI 51.7%-93.2%) and 35.7% (95%CI 16.2%-61.4%), respectively (p=0.054). Similar results were found when analyzing only MPO-ANCA positive patients (6 in the RTX and 9 in the conventional arms), showing that minor and major relapse-free survival rates were 100.0% (95%CI 55.7%-100.0%) and 22.22% (95%CI 5.3%-55.7%), respectively (p=0.007).
Conclusion: Among EGPA patients with active disease, long-term follow-up supports that RTX and the conventional strategy have similar efficacy in inducing and maintaining remission. However, in ANCA-positive patients, RTX was associated with a better relapse-free survival compared to the conventional strategy.
M. Dutertre: None; G. Pugnet: None; C. De Moreuil: None; B. Bonnotte: None; Y. BENHAMOU: None; D. Chauveau: None; E. Diot: None; P. Duffau: None; N. Limal: None; A. Néel: None; G. URBANSKI: None; N. Jourde-Chiche: None; N. MARTIN SILVA: None; F. Maurier: None; A. Mekinian: None; N. Schleinitz: CSL behring, 1, Eusapharma, 6, GSK, 6; F. ackermann: None; A. Fauchais: None; A. Froissart: None; T. Le Gallou: None; Y. Uzunhan: None; J. Viallard: None; A. Berezne: None; l. chiche: None; B. Crestani: None; G. Direz: None; C. DUREL: None; P. Godmer: None; J. Kahn: None; M. Lambert: None; M. de Menthon: None; T. Quemeneur: None; J. Cadranel: None; P. Charles: None; A. Dossier: None; L. Guillevin: None; X. Puéchal: None; B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2.
Background/Purpose: The Rituximab in Eosinophilic Granulomatosis With Polyangiitis (REOVAS) trial compared rituximab (RTX) infusions to conventional strategy for remission-induction in eosinophilic granulomatosis with polyangiitis (EGPA). This trial failed to show a superiority of RTX over the conventional strategy to induce remission at 180 days. However, the long-term efficacy and safety of RTX as compared with conventional strategy in EGPA remains unknown. We report here the long-term results of the REOVAS trial.
Methods: After completion of the 12-month REOVAS trial, patients were followed prospectively, with data on disease activity, medications and adverse events collected by the patients' physicians every 6 months until the last follow-up. The primary endpoint was the minor and major relapse-free survival. Secondary endpoints were major relapses and asthma and ENT exacerbations were also assessed.
Results: Among 105 enrolled patients, only one was lost to follow-up. The median follow-up was 45 months (IQR 34-53).
At month 45 (median follow-up), for the RTX and conventional strategy arms, respectively, the minor and major relapse-free survival rates were 63.5% (95%CI 49.9%-75.2%) and 50.9% (95%CI 37.9%-63.9%) (p=0.24) ; major relapse-free survival rates were 90.4% (95%CI 79.4%-95.8%) and 79.2% (95%CI 66.5%-88.0%) (p=0.17); asthma and/or ENT exacerbation-free survival rates were 42.3% (95%CI 29.9%-55.8%) and 34.0% (95%CI 22.7%-47.4%) (p=0.42) ; and overall EGPA-related event-free survival rates were 32.7% (95%CI 21.5%-46.2%) and 22.6% (95%CI 13.5%-35.5%) (p=0.28). We then focused our analysis on patients with MPO-ANCA and showed that the minor and major relapse-free survival rates were 92.3% (95% CI 66.7% - 99.6%) and 50% (95% CI 58% - 72%) for the RTX and conventional arms, respectively (p=0.02).
As 39 patients were enrolled during follow-up in the double-blind MAINRITSEG trial evaluating RTX versus azathioprine for maintenance in EGPA, we focused our analysis on the 66 patients (63%) who were not enrolled in MAINRITSEG. At month 45, the minor and major relapse-free survival rates for the RTX (n=30) and conventional strategy (n=36) arms were 60.0% (95%CI 42.3%-75.4%) and 38.9% (95%CI 24.8%-55.2%), respectively (p=0.14), and the major relapse-free survival rates were 90.0% (95%CI 73.6%-97.3%) and 72.2% (95%CI 55.9%-84.3%), respectively (p=0.12). When analyzing only ANCA-positive patients (14 in the RTX and 14 in the conventional arms), the minor and major relapse-free survival rates were 78.6% (95%CI 51.7%-93.2%) and 35.7% (95%CI 16.2%-61.4%), respectively (p=0.054). Similar results were found when analyzing only MPO-ANCA positive patients (6 in the RTX and 9 in the conventional arms), showing that minor and major relapse-free survival rates were 100.0% (95%CI 55.7%-100.0%) and 22.22% (95%CI 5.3%-55.7%), respectively (p=0.007).
Conclusion: Among EGPA patients with active disease, long-term follow-up supports that RTX and the conventional strategy have similar efficacy in inducing and maintaining remission. However, in ANCA-positive patients, RTX was associated with a better relapse-free survival compared to the conventional strategy.

Figure 1. Kaplan-Meier curves of the risk of all (major and minor) relapse (A), major relapse (B) in all patients; all relapse (C) and major relapse (D) in MPO-ANCA-positive patients, according to treatment group.
Patients were randomly assigned to receive remission induction therapy with rituximab strategy or conventional strategy, stratified according to disease-flare category, ANCA positivity and the Five Factor score
Patients were randomly assigned to receive remission induction therapy with rituximab strategy or conventional strategy, stratified according to disease-flare category, ANCA positivity and the Five Factor score

Table 1. Baseline characteristics of the patients included in the REOVAS study

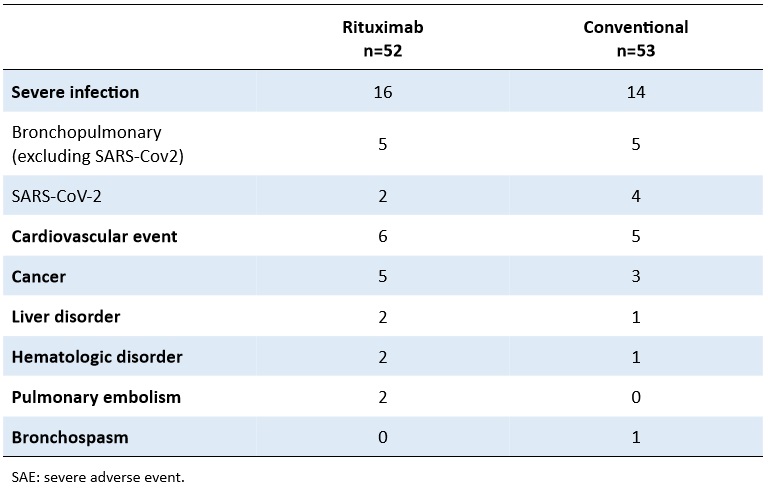
Table 2. Numbers of patients with SAE according to treatment group
M. Dutertre: None; G. Pugnet: None; C. De Moreuil: None; B. Bonnotte: None; Y. BENHAMOU: None; D. Chauveau: None; E. Diot: None; P. Duffau: None; N. Limal: None; A. Néel: None; G. URBANSKI: None; N. Jourde-Chiche: None; N. MARTIN SILVA: None; F. Maurier: None; A. Mekinian: None; N. Schleinitz: CSL behring, 1, Eusapharma, 6, GSK, 6; F. ackermann: None; A. Fauchais: None; A. Froissart: None; T. Le Gallou: None; Y. Uzunhan: None; J. Viallard: None; A. Berezne: None; l. chiche: None; B. Crestani: None; G. Direz: None; C. DUREL: None; P. Godmer: None; J. Kahn: None; M. Lambert: None; M. de Menthon: None; T. Quemeneur: None; J. Cadranel: None; P. Charles: None; A. Dossier: None; L. Guillevin: None; X. Puéchal: None; B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2.



