Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Treatments I: Novel RA Treatments & Mechanisms of Action (0835–0840)
0835: Abatacept in Individuals at Risk of Developing Rheumatoid Arthritis: Results from the Arthritis Prevention in the Pre-clinical Phase of RA with Abatacept (APIPPRA) Trial
Sunday, November 12, 2023
4:00 PM - 4:10 PM PT
Location: Room 6E/6D
- AC
Andrew Cope, MD, PhD
King's College London
London, United KingdomDisclosure information not submitted.
Presenting Author(s)
Andrew Cope1, Marianna Jasenecova2, Joana Vasconcelos3, Andrew Filer4, Karim Raza5, Sumera Qureshi2, Maria Antonietta D'Agostino6, Iain McInnes7, John Isaacs8, Arthur Pratt9, Benjamin A Fisher4, Christopher Buckley10, Paul Emery11, Pauline Ho12, Maya Buch13, Coziana Ciurtin14, René Toes15, Thomas Huizinga15, Dirkjan van Schaardenburg16, Caroline Caroline17 and Toby Prevost3, 1King's College London, London, United Kingdom, 2Centre for Rheumatic Diseases, King's College London, London, United Kingdom, 3Nightingale-Saunders Clinical Trials & Epidemiology Unit, King's College London, London, United Kingdom, 4University of Birmingham, Birmingham, United Kingdom, 5Institute of Inflammation and Ageing, University of Birmingham, Birmingham, United Kingdom, 6Division of Rheumatology - Catholic University of the Sacred Heart, Fondazione Policlinico Universitario Agostino Gemelli, Rome, Italy, 7University of Glasgow, Glasgow, United Kingdom, 8Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, United Kingdom, 9Translational & Clinical Research Institute, University of Newcastle, Newcastle, United Kingdom, 10University of Oxford, Oxford, United Kingdom, 11Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, and NIHR Leeds Biomedical Research Centre, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, 12Centre for Musculoskeletal Research, University of Manchester, Manchester, United Kingdom, 13University of Manchester and NIHR Manchester Biomedical Research Centre, Manchester, United Kingdom, 14Centre for Rheumatology Research, University College London, London, United Kingdom, 15Leiden University Medical Center, Leiden, Netherlands, 16Reade, Amsterdam, Netherlands, 17King's Clinical Trials Unit, King's College London, London, United Kingdom
Background/Purpose: The definition of higher risk states for rheumatoid arthritis (RA) has been refined in more recent years through inclusion of serum autoantibodies and symptom complexes, such as inflammatory joint pain. Data from at risk cohorts have reported rates of progression to RA in excess of 50% over 24 months. These combined features have provided a framework for the design of interception studies, aimed at delaying or preventing RA. We set out to evaluate the feasibility, efficacy and acceptability of T-cell co-stimulation modulation with abatacept in individuals at risk of developing RA in the Arthritis Prevention In the Pre-clinical Phase of RA with Abatacept (APIPPRA) study.
Methods: APIPPRA is a Phase IIB randomised, double blind, placebo-controlled trial recruiting ACPA+RF+ or ACPAhiRF- individuals with arthralgia. Consenting participants were randomised to receive 52 weekly subcutaneous injections of placebo or 125mg abatacept, and followed up for a further 52 weeks. Exclusion criteria included previous episodes of clinical synovitis, prior corticosteroids or DMARDs. The primary endpoint was the time to development of either clinical synovitis in 3 or more joints, or RA according to ACR/EULAR 2010 criteria, whichever was met first. Joint synovitis was confirmed by ultrasonography. Secondary endpoints included multiple disease activity assessments, including ultrasound scores, as well as safety data.
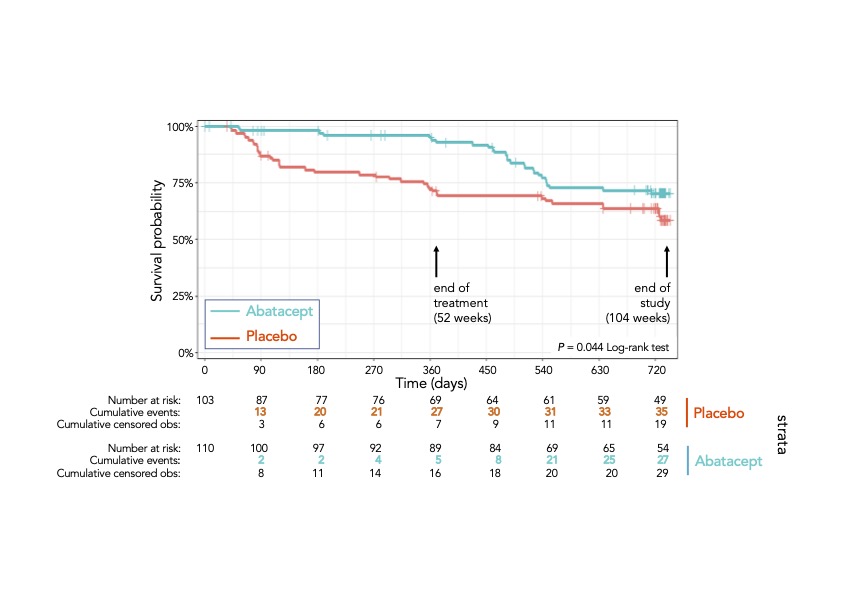
Results: Between December 2014 and January 2019, 280 individuals were evaluated for eligibility across 31 study sites, 28 in the UK and 3 in the Netherlands. Two hundred and thirteen were randomised, 103 to placebo and 110 to abatacept. Mean age was 49 and 77% were female. Ninety-three percent of individuals were ACPAhi. Ultrasonography at baseline suggested modest levels of active sub-clinical synovitis (73% of participants with power Doppler score of 0). In total, there were 65 primary outcome events. At 52 weeks there were 30 events (29%) in the placebo arm and 7 (6%) in the abatacept arm. By the end of the study there were 38 (37%) and 27 (25%) events, respectively, resulting in differences in mean arthritis-free survival time between arms of 99.2 days (95% CI 37.5 – 160.9; p-value=0.002), in favour of abatacept. The respective log-rank test for difference in the survival distribution was p=0.044, reflecting a large effect in the first year and convergence over the second year (see Figure 1). The per protocol analysis showed similar results. Pre-specified exploratory analysis revealed that individuals with high levels of ACPA or who had an extended autoantibody profile at baseline were more likely to remain arthritis-free following abatacept therapy. Those treated with abatacept had lower HAQ-DI and pain scores, and higher EQ-5DL scores during the treatment period, when compared to placebo. There were 4 serious adverse events in the abatacept group and 10 in the placebo group, including two deaths, one in each arm, none deemed attributable to study drug.
Conclusion: Therapeutic intervention during the RA at risk phase is feasible, with acceptable safety profiles. T cell co-stimulation modulation with abatacept for 52 weeks showed a reduction in the development of RA over two years. There were no new safety signals.
A. Cope: Bristol-Myers Squibb(BMS), 2, 5, 6; M. Jasenecova: Bristol-Myers Squibb(BMS), 5; J. Vasconcelos: Bristol-Myers Squibb(BMS), 5; A. Filer: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 5, Janssen, 5, Nascient, 5, Sonoma Biotherapeutics, 2; K. Raza: Bristol-Myers Squibb(BMS), 5; S. Qureshi: Bristol-Myers Squibb(BMS), 5; M. D'Agostino: AbbVie/Abbott, 2, 5, 6, Amgen, 2, 5, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Celgene, 2, 5, 6, Eli Lilly, 2, 5, 6, Janssen, 2, 5, 6, Merck/MSD, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, UCB, 2, 5, 6; I. McInnes: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Bristol Myers Squibb, 2, 5, Cabaletta, 2, 11, Causeway Therapeutics, 2, 11, Celgene, 2, 5, Compugen, 2, 11, Dextera, 11, Eli Lilly, 2, EveloBio, 1, 2, 4, 11, Gilead, 2, Janssen, 2, 5, Moonlake, 2, NHS GGC, 4, Novartis, 2, 5, Pfizer, 2, Sanofi, 2, UCB, 2, 5, Versus Arthritis, 12, Trustee Status; J. Isaacs: Bristol-Myers Squibb(BMS), 5; A. Pratt: Bristol-Myers Squibb(BMS), 5; B. A Fisher: Bristol-Myers Squibb(BMS), 2, Celgene, 5, Galapagos, 2, 5, Janssen, 2, 5, Novartis, 2, Roche, 2, Sanofi, 2, Servier, 2, 5, UCB, 2; C. Buckley: Bristol-Myers Squibb(BMS), 5, Mestag, 11; P. Emery: Boehringer Ingelheim, 2, Eli Lilly, 2, Novartis, 2; P. Ho: Bristol-Myers Squibb(BMS), 5; M. Buch: AbbVie, 2, 6, 12, All paid to host institution, Boehringer Ingelheim, 2, 6, 12, Paid to host institution, Galapagos, 2, 6, 12, Paid to host institution, Gilead, 2, 5, 6, 12, Paid to host institution, Lilly, 2, 6, 12, All paid to host institution, National Insitute for Health and Care Research (NIHR), 3, 12, Maya H Buch is a National Institute for Health and Care Research (NIHR) Senior Investigator. The views expressed are those of the authors and not nece, Pfizer, 2, 12, Paid to host institution; C. Ciurtin: Bristol-Myers Squibb(BMS), 5; R. Toes: Bristol-Myers Squibb(BMS), 5; T. Huizinga: None; D. van Schaardenburg: None; C. Caroline: Bristol-Myers Squibb(BMS), 5; T. Prevost: Bristol-Myers Squibb(BMS), 5.
Background/Purpose: The definition of higher risk states for rheumatoid arthritis (RA) has been refined in more recent years through inclusion of serum autoantibodies and symptom complexes, such as inflammatory joint pain. Data from at risk cohorts have reported rates of progression to RA in excess of 50% over 24 months. These combined features have provided a framework for the design of interception studies, aimed at delaying or preventing RA. We set out to evaluate the feasibility, efficacy and acceptability of T-cell co-stimulation modulation with abatacept in individuals at risk of developing RA in the Arthritis Prevention In the Pre-clinical Phase of RA with Abatacept (APIPPRA) study.
Methods: APIPPRA is a Phase IIB randomised, double blind, placebo-controlled trial recruiting ACPA+RF+ or ACPAhiRF- individuals with arthralgia. Consenting participants were randomised to receive 52 weekly subcutaneous injections of placebo or 125mg abatacept, and followed up for a further 52 weeks. Exclusion criteria included previous episodes of clinical synovitis, prior corticosteroids or DMARDs. The primary endpoint was the time to development of either clinical synovitis in 3 or more joints, or RA according to ACR/EULAR 2010 criteria, whichever was met first. Joint synovitis was confirmed by ultrasonography. Secondary endpoints included multiple disease activity assessments, including ultrasound scores, as well as safety data.
Results: Between December 2014 and January 2019, 280 individuals were evaluated for eligibility across 31 study sites, 28 in the UK and 3 in the Netherlands. Two hundred and thirteen were randomised, 103 to placebo and 110 to abatacept. Mean age was 49 and 77% were female. Ninety-three percent of individuals were ACPAhi. Ultrasonography at baseline suggested modest levels of active sub-clinical synovitis (73% of participants with power Doppler score of 0). In total, there were 65 primary outcome events. At 52 weeks there were 30 events (29%) in the placebo arm and 7 (6%) in the abatacept arm. By the end of the study there were 38 (37%) and 27 (25%) events, respectively, resulting in differences in mean arthritis-free survival time between arms of 99.2 days (95% CI 37.5 – 160.9; p-value=0.002), in favour of abatacept. The respective log-rank test for difference in the survival distribution was p=0.044, reflecting a large effect in the first year and convergence over the second year (see Figure 1). The per protocol analysis showed similar results. Pre-specified exploratory analysis revealed that individuals with high levels of ACPA or who had an extended autoantibody profile at baseline were more likely to remain arthritis-free following abatacept therapy. Those treated with abatacept had lower HAQ-DI and pain scores, and higher EQ-5DL scores during the treatment period, when compared to placebo. There were 4 serious adverse events in the abatacept group and 10 in the placebo group, including two deaths, one in each arm, none deemed attributable to study drug.
Conclusion: Therapeutic intervention during the RA at risk phase is feasible, with acceptable safety profiles. T cell co-stimulation modulation with abatacept for 52 weeks showed a reduction in the development of RA over two years. There were no new safety signals.

A. Cope: Bristol-Myers Squibb(BMS), 2, 5, 6; M. Jasenecova: Bristol-Myers Squibb(BMS), 5; J. Vasconcelos: Bristol-Myers Squibb(BMS), 5; A. Filer: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 5, Janssen, 5, Nascient, 5, Sonoma Biotherapeutics, 2; K. Raza: Bristol-Myers Squibb(BMS), 5; S. Qureshi: Bristol-Myers Squibb(BMS), 5; M. D'Agostino: AbbVie/Abbott, 2, 5, 6, Amgen, 2, 5, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Celgene, 2, 5, 6, Eli Lilly, 2, 5, 6, Janssen, 2, 5, 6, Merck/MSD, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, UCB, 2, 5, 6; I. McInnes: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Bristol Myers Squibb, 2, 5, Cabaletta, 2, 11, Causeway Therapeutics, 2, 11, Celgene, 2, 5, Compugen, 2, 11, Dextera, 11, Eli Lilly, 2, EveloBio, 1, 2, 4, 11, Gilead, 2, Janssen, 2, 5, Moonlake, 2, NHS GGC, 4, Novartis, 2, 5, Pfizer, 2, Sanofi, 2, UCB, 2, 5, Versus Arthritis, 12, Trustee Status; J. Isaacs: Bristol-Myers Squibb(BMS), 5; A. Pratt: Bristol-Myers Squibb(BMS), 5; B. A Fisher: Bristol-Myers Squibb(BMS), 2, Celgene, 5, Galapagos, 2, 5, Janssen, 2, 5, Novartis, 2, Roche, 2, Sanofi, 2, Servier, 2, 5, UCB, 2; C. Buckley: Bristol-Myers Squibb(BMS), 5, Mestag, 11; P. Emery: Boehringer Ingelheim, 2, Eli Lilly, 2, Novartis, 2; P. Ho: Bristol-Myers Squibb(BMS), 5; M. Buch: AbbVie, 2, 6, 12, All paid to host institution, Boehringer Ingelheim, 2, 6, 12, Paid to host institution, Galapagos, 2, 6, 12, Paid to host institution, Gilead, 2, 5, 6, 12, Paid to host institution, Lilly, 2, 6, 12, All paid to host institution, National Insitute for Health and Care Research (NIHR), 3, 12, Maya H Buch is a National Institute for Health and Care Research (NIHR) Senior Investigator. The views expressed are those of the authors and not nece, Pfizer, 2, 12, Paid to host institution; C. Ciurtin: Bristol-Myers Squibb(BMS), 5; R. Toes: Bristol-Myers Squibb(BMS), 5; T. Huizinga: None; D. van Schaardenburg: None; C. Caroline: Bristol-Myers Squibb(BMS), 5; T. Prevost: Bristol-Myers Squibb(BMS), 5.



