Abstract Session
Periodic fever syndromes, autoinflammatory diseases, Still’s disease and MAS/HLH
Session: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases I (0757–0762)
0762: Liver Disease Is a Common Feature of HA20 That Causes Significant Morbidity Associated with Interferon Induction
Sunday, November 12, 2023
3:15 PM - 3:25 PM PT
Location: Room 5A-B
- MH
Magdalena Harasimowicz, MD
University of Pittsburgh Medical Center (UPMC)
Pittsburgh, PA, United StatesDisclosure information not submitted.
Presenting Author(s)
Magdalena Harasimowicz1, Deborah Stone2, Manuel Carpio Tumba1, Tina Romeo2, Urekha Karri1, Patrycja Hoffmann3, Helen Leavis4, Alexander Miethke5, Theo Heller2, Anjali Rai2, Ivona Aksentijevich2, amanda ombrello6, Daniel Kastner7 and Daniella Schwartz1, 1University of Pittsburgh, Pittsburgh, PA, 2NIH, Bethesda, MD, 3NIH, Vienna, VA, 4Infection Immunity Utrecht, Utrecht, Netherlands, 5Cincinnati Children's Hospital, Cincinnati, OH, 6National Institutes of Health, Rockville, MD, 7National Human Genome Research Institute, Bethesda, MD
Background/Purpose: Heterozygous loss-of-function TNFAIP3 mutations cause A20 haploinsufficiency (HA20), an early-onset immune dysregulatory disease1. While HA20 was initially described as an inherited form of Behcet’s syndrome, it is now known to cause wider range of phenotypes including psoriasis, lymphoproliferation, membranous nephropathy, and liver injury.2 Prior work suggests that liver disease is seen in only 8% of HA20 patients.3-7 Contrary to this paradigm, we noted a high prevalence of liver injury when evaluating a cross-section of 41 HA20 patients by virtual consultation. We therefore systematically assessed our cohort of 25 HA20 patients, finding an unexpectedly high prevalence of liver disease with significant morbidity.
Methods: 41 HA20 patients from 5 countries were referred for virtual consultation. 24 of these patents were evaluated at the NIH Clinical Center or at UPMC; 1 subject was deceased at time of assessment but had been evaluated at the referring institution. Clinical laboratory and imaging data were obtained via chart review. Interferon stimulated genes (ISGs) were measured in whole blood (Nanostring) from 15 patients. Fisher’s t-test with multiple comparison adjustment (Benjamini-Hochberg) was used to compare patients with vs. without liver disease.
Results: Liver disease was described in 19 virtual consultation patients (44%). 12 (29.3%) were diagnosed with autoimmune hepatitis, 2 of whom (4.9%) died from complications of HA20. Serial laboratory results were available for all 25 retrospectively evaluated patients, abdominal imaging was available for 14, and liver biopsies had been done in 6. 12 patients (48%) had liver disease: 8 (29.2%) were diagnosed clinically, and 4 occult cases were found on abdominal imaging.
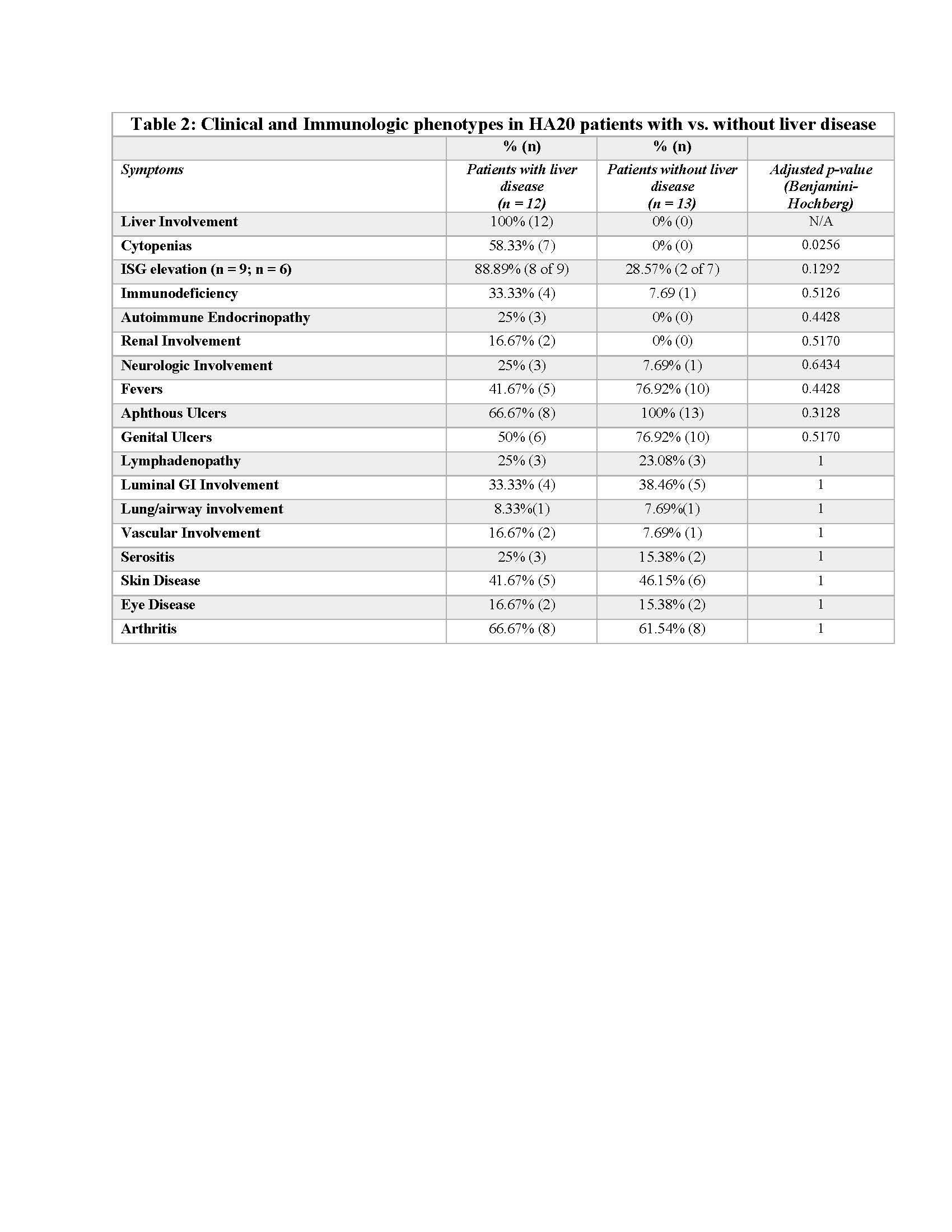
Mean age of onset was 11.2. Mutations involved various domains of A20; the entire coding sequence was deleted in 4 patients from 2 families (Figure 1). 6 patients were diagnosed with autoimmune hepatitis, 1 had recurrent transaminase elevation ( >3x upper limit of normal), 2 had hepatic steatosis, and 3 had isolated hepatomegaly (Table 1). Fibroscan scores ranged from 2.7–22.7 kPa. 6 patients had biopsy-proven fibrosis: 4 had cirrhosis and 3 had portal hypertension. ISGs were checked in 9 patients and elevated in 8 (88.8%). 4 patients (33.3%) complete or partial prior response to azathioprine (AZA); 6 (50%) patients had complete or partial prior response to JAK inhibitors (JAKi). Compared with the rest of the cohort (n = 13), patients with liver disease had significantly more cytopenias (Table 2). There was a trend towards more immunodeficiency, more autoimmunity, more ISG induction, and fewer Behcet’s-like features.
Conclusion: Hepatitis is highly prevalent in HA20 and has a substantial morbidity and mortality. Occult steatosis and hepatomegaly are common; longitudinal studies are needed to determine the significance of these features. We recommend screening all HA20 patients periodically (hepatic panel, ultrasound) and referring patients with abnormal findings to hepatology. In HA20 patients with liver disease, we recommend AZA as a reasonable first-line treatment. JAK inhibitors may be effective in patients with incomplete responses to AZA.
.jpg)
.jpg)
M. Harasimowicz: None; D. Stone: None; M. Carpio Tumba: None; T. Romeo: None; U. Karri: None; P. Hoffmann: None; H. Leavis: None; A. Miethke: Mirum Pharmaceuticals, 5; T. Heller: None; A. Rai: None; I. Aksentijevich: None; a. ombrello: None; D. Kastner: None; D. Schwartz: None.
Background/Purpose: Heterozygous loss-of-function TNFAIP3 mutations cause A20 haploinsufficiency (HA20), an early-onset immune dysregulatory disease1. While HA20 was initially described as an inherited form of Behcet’s syndrome, it is now known to cause wider range of phenotypes including psoriasis, lymphoproliferation, membranous nephropathy, and liver injury.2 Prior work suggests that liver disease is seen in only 8% of HA20 patients.3-7 Contrary to this paradigm, we noted a high prevalence of liver injury when evaluating a cross-section of 41 HA20 patients by virtual consultation. We therefore systematically assessed our cohort of 25 HA20 patients, finding an unexpectedly high prevalence of liver disease with significant morbidity.
Methods: 41 HA20 patients from 5 countries were referred for virtual consultation. 24 of these patents were evaluated at the NIH Clinical Center or at UPMC; 1 subject was deceased at time of assessment but had been evaluated at the referring institution. Clinical laboratory and imaging data were obtained via chart review. Interferon stimulated genes (ISGs) were measured in whole blood (Nanostring) from 15 patients. Fisher’s t-test with multiple comparison adjustment (Benjamini-Hochberg) was used to compare patients with vs. without liver disease.
Results: Liver disease was described in 19 virtual consultation patients (44%). 12 (29.3%) were diagnosed with autoimmune hepatitis, 2 of whom (4.9%) died from complications of HA20. Serial laboratory results were available for all 25 retrospectively evaluated patients, abdominal imaging was available for 14, and liver biopsies had been done in 6. 12 patients (48%) had liver disease: 8 (29.2%) were diagnosed clinically, and 4 occult cases were found on abdominal imaging.
Mean age of onset was 11.2. Mutations involved various domains of A20; the entire coding sequence was deleted in 4 patients from 2 families (Figure 1). 6 patients were diagnosed with autoimmune hepatitis, 1 had recurrent transaminase elevation ( >3x upper limit of normal), 2 had hepatic steatosis, and 3 had isolated hepatomegaly (Table 1). Fibroscan scores ranged from 2.7–22.7 kPa. 6 patients had biopsy-proven fibrosis: 4 had cirrhosis and 3 had portal hypertension. ISGs were checked in 9 patients and elevated in 8 (88.8%). 4 patients (33.3%) complete or partial prior response to azathioprine (AZA); 6 (50%) patients had complete or partial prior response to JAK inhibitors (JAKi). Compared with the rest of the cohort (n = 13), patients with liver disease had significantly more cytopenias (Table 2). There was a trend towards more immunodeficiency, more autoimmunity, more ISG induction, and fewer Behcet’s-like features.
Conclusion: Hepatitis is highly prevalent in HA20 and has a substantial morbidity and mortality. Occult steatosis and hepatomegaly are common; longitudinal studies are needed to determine the significance of these features. We recommend screening all HA20 patients periodically (hepatic panel, ultrasound) and referring patients with abnormal findings to hepatology. In HA20 patients with liver disease, we recommend AZA as a reasonable first-line treatment. JAK inhibitors may be effective in patients with incomplete responses to AZA.
.jpg)
Figure 1. TNFAIP3 mutations in patients with and without liver injury. Liver disease associated mutations involve multiple domains of HA20, including deletion of the entire coding sequence
.jpg)
Table 1. Features of HA20 patients with liver disease. AIH: autoimmune hepatitis, CS: corticosteroids; AZA: azathioprine, ISG: interferon stimulated genes, ITP: Immune thrombocytopenic purpura, JAKi: Janus Kinase Inhibitor, N/A: not applicable, N/C: not checked

Table 2. Clinical and Immunologic phenotypes in HA20 patients with vs. without liver disease. ISG: interferon stimulated genes, GI: gastrointestinal
M. Harasimowicz: None; D. Stone: None; M. Carpio Tumba: None; T. Romeo: None; U. Karri: None; P. Hoffmann: None; H. Leavis: None; A. Miethke: Mirum Pharmaceuticals, 5; T. Heller: None; A. Rai: None; I. Aksentijevich: None; a. ombrello: None; D. Kastner: None; D. Schwartz: None.



